Long-term reduction in morning and nighttime blood pressure after renal denervation: 36-month results from SPYRAL HTN-ON MED trial
- PMID: 36241705
- PMCID: PMC9747613
- DOI: 10.1038/s41440-022-01042-8
Long-term reduction in morning and nighttime blood pressure after renal denervation: 36-month results from SPYRAL HTN-ON MED trial
Abstract
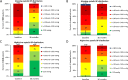
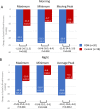
Elevated morning and nighttime blood pressures (BP) are associated with increased risk of cardiovascular events such as stroke and myocardial infarction. We compared the long-term changes in morning and nighttime BP in patients with uncontrolled hypertension (office systolic BP between 150 and <180 mmHg/diastolic BP ≥ 90 mmHg; mean ambulatory systolic BP (SBP) between 140 and <170 mmHg; 1-3 prescribed antihypertensive medications). Eighty patients were randomized to RDN or sham control. In patients taking at least 3 antihypertensive medications at 36 months (N = 23 RDN group; N = 23 sham group), the 24 h ambulatory SBP as well as morning (7:00-9:00AM) and nighttime (1:00-6:00AM) ambulatory SBP were significantly lower for the RDN group compared to sham control (24 h SBP: -20.2 vs. -10.2, p = 0.0087; morning SBP: -23.9 vs. -8.0 mmHg, p = 0.029; nighttime SBP: -20.8 vs. -7.2 mmHg, p = 0.0011). At 36 months, 24 h SBP was controlled to <130 mmHg in 40% of RDN patients in the morning compared to 6% for the sham group; P = 0.021 and in 80% of the RDN patients at night compared to 39% in the sham group; P = 0.019. Major adverse events through 36 months were rare in both groups, and there were no renal artery re-interventions or vascular complications. Morning and nighttime SBP were significantly lower in patients prescribed at least 3 antihypertensive medications at 36 months in the SPYRAL HTN-ON MED trial for RDN compared with sham control. The results suggest RDN has significant benefit when the risk of cardiovascular events is highest.
Keywords: Cardiovascular prognosis; Randomized controlled trial; Renal denervation; Resistant hypertension.
© 2022. The Author(s).
Conflict of interest statement
Prof KK receives personal fees from Medtronic during the conduct of the study. He reports research funding from Otsuka holdings; honoraria from Otsuka Medical Device, JIMRO and Terumo, outside the submitted work. Prof FM is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219), and Deutsche Herzstiftung. He has received scientific support from Medronic and ReCor Medical and speaker honoraria from Astra-Zeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, and ReCor Medical. Dr DEK reports institutional research/grant support from Biotronik, Boston Scientific, Cardiovascular Systems, Inc., Orbus Neich, Teleflex, Medtronic and Ablative Solutions; and personal consulting honoraria from Ablative Solutions, Cardiovascular Systems, Inc., Magenta Medical, Medtronic, and Terumo. Prof RRT is a consultant for Medtronic, AXIO and Janssen, and receives royalties from UpToDate. Prof MAW is a consultant for Medtronic, ReCor, Ablative Solutions, Johnson & Johnson and Urovant. Prof RES reports grants and personal fees from Medtronic, Recor, and Ablative Solutions. Prof KT has received scientific support and speaker honoraria from Servier, Novartis,Astra-Zeneca, Bayer, Boehringer Ingelheim, Amgen, Pfizer, Viatris, Menarini, Medtronic and ReCor Medical. Dr SP reports personal fees from Medtronic outside the submitted work. Dr SB, Dr DAH, and Mr MF are employees of Medtronic. Prof MB is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR 219, project number 322900939) and reports personal fees from Abbott, Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Medtronic, Novartis, ReCor Medical, Servier, and Vifor during the conduct of the study.
Figures


Comment in
-
Is renal denervation a natural antihypertensive treatment?Hypertens Res. 2023 Jan;46(1):289-290. doi: 10.1038/s41440-022-01109-6. Epub 2022 Nov 15. Hypertens Res. 2023. PMID: 36380205 No abstract available.
References
-
- Kario K, Hoshide S, Mizuno H, Kabutoya T, Nishizawa M, Yoshida T, et al. Nighttime hemodynamic phenotype. A novel risk factor for cardiovascular disease, especially heart failure: the practitioner-based nationwide JAMP study. Clin Res Cardiol. 2022. 10.1007/s00392-022-02051-w. [Epub ahead of print]. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

