Design and function of targeted endocannabinoid nanoparticles
- PMID: 36241847
- PMCID: PMC9568518
- DOI: 10.1038/s41598-022-21715-1
Design and function of targeted endocannabinoid nanoparticles
Abstract
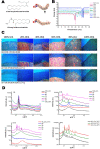
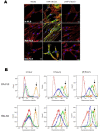
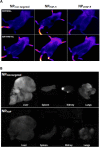
Nanoparticles and nano-delivery systems are constantly being refined and developed for biomedical applications such as imaging, gene therapy, and targeted delivery of drugs. Nanoparticles deliver beneficial effects by both release of their cargo and by liberation of their constitutive structural components. The N-acylethanolamines linoleoyl ethanolamide (LEA) and oleoyl ethanolamide (OEA) both exhibit endocannabinoid-like activity. Here, we report on their ability to form nanoparticles that when conjugated with tissue-specific molecules, are capable of localizing to specific areas of the body and reducing inflammation. The facilitation of pharmacological effects by endocannabinoids at targeted sites provides a novel biocompatible drug delivery system and a therapeutic approach to the treatment, patient management and quality of life, in conditions such as arthritis, epilepsy, and cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict or competing interests. No financial disclosures. Ms. N Barrie was funded by a Rheumatology Dept. Scholarship.
Figures



References
-
- Gonsiorek W, et al. Endocannabinoid 2-arachidonyl glycerol is a full agonist through human type 2 cannabinoid receptor: Antagonism by anandamide. Mol. Pharmacol. 2000;57:1045–1050. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

