In vitro chemotherapy-associated muscle toxicity is attenuated with nutritional support, while treatment efficacy is retained
- PMID: 36242541
- PMCID: PMC9564364
- DOI: 10.18632/oncotarget.28279
In vitro chemotherapy-associated muscle toxicity is attenuated with nutritional support, while treatment efficacy is retained
Abstract
Purpose: Muscle-wasting and treatment-related toxicities negatively impact prognosis of colorectal cancer (CRC) patients. Specific nutritional composition might support skeletal muscle and enhance treatment support. In this in vitro study we assess the effect of nutrients EPA, DHA, L-leucine and vitamin D3, as single nutrients or in combination on chemotherapy-treated C2C12-myotubes, and specific CRC-tumor cells.
Materials and methods: Using C2C12-myotubes, the effects of chemotherapy (oxaliplatin, 5-fluorouracil, oxaliplatin+5-fluorouracil and irinotecan) on protein synthesis, cell-viability, caspase-3/7-activity and LDH-activity were assessed. Addition of EPA, DHA, L-leucine and vitamin D3 and their combination (SNCi) were studied in presence of above chemotherapies. Tumor cell-viability was assessed in oxaliplatin-treated C26 and MC38 CRC cells, and in murine and patient-derived CRC-organoids.
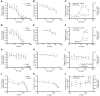
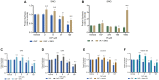
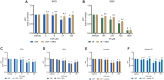
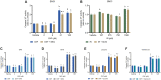
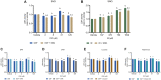
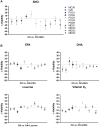
Results: While chemotherapy treatment of C2C12-myotubes decreased protein synthesis, cell-viability and increased caspase-3/7 and LDH-activity, SNCi showed improved protein synthesis and cell viability and lowered LDH activity. The nutrient combination SNCi showed a better overall performance compared to the single nutrients. Treatment response of tumor models was not significantly affected by addition of nutrients.
Conclusions: This in vitro study shows protective effect with specific nutrition composition of C2C12-myotubes against chemotherapy toxicity, which is superior to the single nutrients, while treatment response of tumor cells remained.
Keywords: C2C12 myotubes; chemotherapy; chemotherapy-associated toxicity; colorectal cancer; nutrients; tumor (organoid) cells.
Conflict of interest statement
All authors have no relevant conflicts of interest to disclose. Francina Dijk, Hanil Quirindongo, Mathew Furber, Bram Dorresteijn and Miriam van Dijk are employees of Danone Nutricia Research, Utrecht, The Netherlands. Liza Wijler, Danielle Raats, Anne May and Onno Kranenburg are employees of University Medical Center Utrecht, Utrecht, The Netherlands.
Figures

References
-
- Anker MS, Holcomb R, Muscaritoli M, von Haehling S, Haverkamp W, Jatoi A, Morley JE, Strasser F, Landmesser U, Coats AJS, Anker SD. Orphan disease status of cancer cachexia in the USA and in the European Union: a systematic review. J Cachexia Sarcopenia Muscle. 2019; 10:22–34. 10.1002/jcsm.12402. - DOI - PMC - PubMed
-
- Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, Jatoi A, Loprinzi C, MacDonald N, Mantovani G, Davis M, Muscaritoli M, Ottery F, et al.. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011; 12:489–95. 10.1016/S1470-2045(10)70218-7. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

