The Safety of Deutetrabenazine for Chorea in Huntington Disease: An Open-Label Extension Study
- PMID: 36242718
- PMCID: PMC9653309
- DOI: 10.1007/s40263-022-00956-8
The Safety of Deutetrabenazine for Chorea in Huntington Disease: An Open-Label Extension Study
Abstract
Background: Deutetrabenazine is approved in the USA, China, Australia, Israel, Brazil, and South Korea for the treatment of chorea associated with Huntington disease.
Objective: We aimed to evaluate the long-term safety and tolerability of deutetrabenazine for the treatment of Huntington disease.
Methods: This open-label, single-arm, multi-center study included patients who completed a double-blind study (Rollover) and patients who converted overnight from a stable tetrabenazine dose (Switch). Exposure-adjusted incidence rates (adverse events per person-year) were calculated. Efficacy was analyzed using a stable post-titration timepoint (8 weeks). Changes in the Unified Huntington's Disease Rating Scale total motor score and total maximal chorea score from baseline to week 8, as well as those from week 8 to week 145 (or the last visit on the study drug if that occurred earlier), were evaluated as both efficacy and safety endpoints during the study.
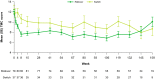
Results: Of 119 patients (Rollover, n = 82; Switch, n = 37), 100 (84%) completed ≥ 1 year of treatment. End-of-study exposure-adjusted incidence rates for adverse events in Rollover and Switch, respectively, were: any, 2.57 and 4.02; serious, 0.11 and 0.14; leading to dose suspension, 0.05 and 0.04. Common adverse events (≥ 4% either cohort) included somnolence (Rollover, 20%; Switch, 30%), depression (32%; 22%), anxiety (27%; 35%), insomnia (23%; 16%), and akathisia (6%; 11%). Adverse events of interest included suicidality (9%; 5%) and parkinsonism (4%; 8%). Mean dose at week 8 was 38.1 mg (Rollover) and 36.5 mg (Switch). Mean dose across cohorts after titration was 37.6 mg; at the final visit, mean dose across cohorts was 45.7 mg. Patients showed minimal change in the Unified Huntington's Disease Rating Scale total maximal chorea scores with stable dosing from weeks 8-145 or at the end of treatment, but total motor score increased versus week 8 (mean change [standard deviation]: 8.2 [11.9]). There were no unexpected adverse events upon drug withdrawal, and mean (standard deviation) total maximal chorea scores increased 4.7 (4.6) units from week 8 to 1-week follow-up.
Conclusions: Adverse events observed with long-term deutetrabenazine exposure were consistent with previous studies. Reductions in chorea persisted over time. Upon treatment cessation, there was no unexpected worsening of chorea.
Clinical trial registration: ClinicalTrials.gov identifier: NCT01897896.
© 2022. The Author(s).
Conflict of interest statement
Samuel Frank and Claudia Testa served as co-principal investigators of First-HD. Claudia Testa also served as a consultant for Lundbeck, with honoraria donated to the Medical College of Virginia Foundation. Mary C. Edmondson reports serving on a Wave Life Sciences Clinical Advisory Committee, funding from uniQure, and ENROLL-HD DSMB involvement. Blair R. Leavitt is currently employed as a Professor in the Department of Medical Genetics, and Division of Neurology, Department of Medicine and as a senior scientist at the Centre for Molecular Medicine and Therapeutics, The University of British Columbia, and BC Children’s Hospital. In the last 5 years, he has held or applied for research grants in the area of neurodegenerative disease from the Canadian Institutes of Health Research, The Huntington Society of Canada, Weston Brain Foundation, Brain Canada, uniQure, Triplet, and the Nanomedicines Innovations Network. He has served on scientific advisory boards for sRNAlytics, the Huntington’s Disease Society of America, and the Huntington Society of Canada. In addition, he has acted as a paid consultant to Roche, uniQure, Novartis, PTC Therapeutics, Triplet Therapeutics, Genentech, Takeda, and Ionis. He is also a shareholder, co-founder, and CEO of Incisive Genetics Inc., a biotech start-up company developing therapies for genetic brain diseases. David Oakes received research support from Auspex (for the First-HD and related studies), Vaccinex Inc., Prana Pharmaceuticals, Biogen, Inc., and the National Institutes of Health; and received honoraria from Raptor Pharmaceuticals and Voyager Inc. Christina Vaughan received an emergency fast-track COVID-19 response grant from the Adira Foundation. Nicholas Gross and Mark Forrest Gordon are employees of Teva Pharmaceuticals, and Juha-Matti Savola is a former employee of Teva Pharmaceuticals. Jody Goldstein, Elise Kayson, Christine O’Neill, and Jacquelyn Whaley report no disclosures.
Qualified researchers may request access to patient-level data and related study documents, including the study protocol and the statistical analysis plan. Requests will be reviewed for scientific merit, product approval status, and conflicts of interest. Patient-level data will be de-identified and study documents will be redacted to protect the privacy of trial participants and to protect commercially confidential information. Please e-mail USMedInfo@tevapharm.com to make your request.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical