The challenge of recruiting multimorbid older patients identified in a hospital database to a randomised controlled trial
- PMID: 36242723
- PMCID: PMC9719448
- DOI: 10.1007/s40520-022-02263-0
The challenge of recruiting multimorbid older patients identified in a hospital database to a randomised controlled trial
Abstract
Background: Research involving multimorbid older patients is gaining momentum. However, little is known about how to plan a randomised controlled trial (RCT) involving this group of patients. An evidence-based approach to the challenges of a recruitment process could guide researchers and help prevent underpowered trials.
Aim: To define the number of multimorbid older patients that need to be identified and the number of eligible patients that need to be invited to achieve the desired recruitment number to a RCT.
Method: We used recruitment data from the GerMoT trial, a RCT comparing proactive outpatient care based on Comprehensive Geriatric Assessment with usual care. Multimorbid older patients with high healthcare utilisation were recruited to the trial.
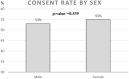
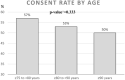
Results: Of the 1212 patients identified in a database as meeting the inclusion criteria 838 (70%) could be invited to participate in the trial. The rest could not be invited for a variety of reasons; 162 had moved out of area or into nursing homes and 86 had died before they could be contacted. 113 could not be reached. 450 (54%) of the invited patients agreed to participate.
Conclusions: In our study, we have shown that it is possible to achieve a good consent rate despite older participants with multimorbidity. This can be used when planning an RCT for this patient group, who are often excluded from clinical trials. Our results are specific to a context that provides similar abilities to identify and recruit patients as can be seen in Sweden.
Keywords: Consent rate; Elderly; Multimorbid; Randomised controlled trial; Recruitment.
© 2022. The Author(s).
Conflict of interest statement
The authors have no conflict of interest to declare that are relevant to the content of this article.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

