Thrombopoietin participates in platelet activation in COVID-19 patients
- PMID: 36242922
- PMCID: PMC9556163
- DOI: 10.1016/j.ebiom.2022.104305
Thrombopoietin participates in platelet activation in COVID-19 patients
Abstract
Background: The pathogenesis of coronavirus disease 2019 (COVID-19) is characterized by enhanced platelet activation and diffuse hemostatic alterations, which may contribute to immunothrombosis/thromboinflammation and subsequent development of target-organ damage. Thrombopoietin (THPO), a growth factor essential to megakariocyte proliferation, is known to prime platelet activation and leukocyte-platelet interaction. In addition, THPO concentrations increase in several critical diseases, such as acute cardiac ischemia and sepsis, thus representing a potential diagnostic and prognostic biomarker. Furthermore, several data suggest that interleukin (IL)-6 is one of the most important inflammatory mediators involved in these phenomena, which led to explore the potential therapeutic role of IL-6 inhibitors. In this prospective cohort study, we aimed to study THPO and IL-6 concentrations in COVID-19 patients at the time of first clinical evaluation in the Emergency Department (ED), and to investigate their potential use as diagnostic and prognostic biomarkers. In addition, we sought to explore the role of THPO contained in plasma samples obtained from COVID-19 patients in priming in vitro platelet activation and leukocyte-platelet interaction.
Methods: We enrolled 66 patients presenting to the ED with symptoms suggestive of COVID-19, including 47 with confirmed COVID-19 and 19 in whom COVID-19 was excluded (Non-COVID-19 patients). As controls, we also recruited 18 healthy subjects. In vitro, we reproduced the effects of increased circulating THPO on platelet function by adding plasma from COVID-19 patients or controls to platelet-rich plasma or whole blood obtained by healthy donors, and we indirectly studied the effect of THPO on platelet activation by blocking its biological activity.
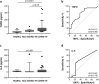
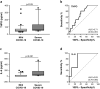
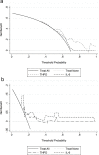
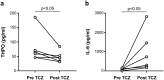
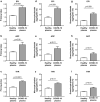
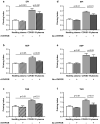
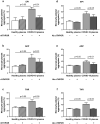
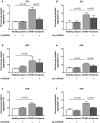
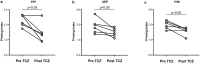
Findings: THPO levels were higher in COVID-19 patients than in both Non-COVID-19 patients and healthy subjects. Studying THPO as diagnostic marker for the diagnosis of COVID-19 by receiver-operating-characteristic (ROC) statistics, we found an area under the curve (AUC) of 0.73, with an optimal cut-off value of 42.60 pg/mL. IL-6 was higher in COVID-19 patients than in healthy subjects, but did not differ between COVID-19 and Non-COVID-19 patients. THPO concentrations measured at the time of diagnosis in the ED were also higher in COVID-19 patients subsequently developing a severe disease than in those with mild disease. Evaluating THPO as biomarker for severe COVID-19 using ROC analysis, we found an AUC of 0.71, with an optimal cut-off value of 57.11 pg/mL. IL-6 was also higher in severe than in mild COVID-19 patients, with an AUC for severe COVID-19 of 0.83 and an optimal cut-off value of 23 pg/ml. THPO concentrations correlated with those of IL-6 (r=0.2963; p=0.043), and decreased 24 h after the administration of tocilizumab, an IL-6 receptor blocking antibody, showing that the increase of THPO levels depends on IL-6-stimulated hepatic synthesis. In vitro, plasma obtained from COVID-19 patients, but not from healthy subjects, primed platelet aggregation and leukocyte-platelet binding, and these effects were reduced by inhibiting THPO activity.
Interpretation: Increased THPO may be proposed as an early biomarker for the diagnosis of COVID-19 and for the identification of patients at risk of developing critical illness. Elevated THPO may contribute to enhance platelet activation and leukocyte-platelet interaction in COVID-19 patients, thus potentially participating in immunothrombosis/thromboinflammation.
Funding: This work was supported by Ministero dell'Università e della Ricerca Scientifica e Tecnologica (MURST) ex 60% to GM and EL.
Keywords: Biomarker; COVID-19; Interleukin-6; Platelet activation; Thromboinflammation; Thrombopoietin.
Copyright © 2022 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Authors declare that no conflict of interest exists.
Figures
Similar articles
-
Thrombopoietin Contributes to Enhanced Platelet Activation in Patients with Type 1 Diabetes Mellitus.Int J Mol Sci. 2021 Jun 29;22(13):7032. doi: 10.3390/ijms22137032. Int J Mol Sci. 2021. PMID: 34210000 Free PMC article.
-
Hepatic thrombopoietin gene silencing reduces platelet count and breast cancer progression in transgenic MMTV-PyMT mice.Blood Adv. 2019 Oct 22;3(20):3080-3091. doi: 10.1182/bloodadvances.2019000250. Blood Adv. 2019. PMID: 31648335 Free PMC article.
-
Complement activation predicts negative outcomes in COVID-19: The experience from Northen Italian patients.Autoimmun Rev. 2023 Jan;22(1):103232. doi: 10.1016/j.autrev.2022.103232. Epub 2022 Nov 19. Autoimmun Rev. 2023. PMID: 36414219 Free PMC article. Review.
-
Elevated thrombopoietin in plasma of burned patients without and with sepsis enhances platelet activation.J Thromb Haemost. 2009 Jun;7(6):1000-8. doi: 10.1111/j.1538-7836.2009.03348.x. Epub 2009 Mar 20. J Thromb Haemost. 2009. PMID: 19317837
-
Thrombopoietin as biomarker and mediator of cardiovascular damage in critical diseases.Mediators Inflamm. 2012;2012:390892. doi: 10.1155/2012/390892. Epub 2012 Apr 5. Mediators Inflamm. 2012. PMID: 22577249 Free PMC article. Review.
Cited by
-
Extracellular Vesicles: New Players in the Mechanisms of Sepsis- and COVID-19-Related Thromboinflammation.Int J Mol Sci. 2023 Jan 18;24(3):1920. doi: 10.3390/ijms24031920. Int J Mol Sci. 2023. PMID: 36768242 Free PMC article. Review.
-
Dynamic changes in platelet counts and psychological state in ITP patients after COVID-19 infection.Front Med (Lausanne). 2025 Mar 14;12:1485418. doi: 10.3389/fmed.2025.1485418. eCollection 2025. Front Med (Lausanne). 2025. PMID: 40160330 Free PMC article.
-
Distinct type 1 immune networks underlie the severity of restrictive lung disease after COVID-19.Nat Immunol. 2025 Apr;26(4):595-606. doi: 10.1038/s41590-025-02110-0. Epub 2025 Mar 26. Nat Immunol. 2025. PMID: 40140496
-
The evolution of preexisting primary immune thrombocytopenia after COVID-19 onset: A nationally representative, prospective, multicentre, observational study.Ann Hematol. 2024 May;103(5):1549-1559. doi: 10.1007/s00277-024-05720-0. Epub 2024 Mar 25. Ann Hematol. 2024. PMID: 38526649
-
Platelet-neutrophil interaction in COVID-19 and vaccine-induced thrombotic thrombocytopenia.Front Immunol. 2023 May 19;14:1186000. doi: 10.3389/fimmu.2023.1186000. eCollection 2023. Front Immunol. 2023. PMID: 37275917 Free PMC article. Review.
References
-
- Wu Z, McGoogan JM. Characteristics of and Important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the Chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. - PubMed
-
- Pellegrini D, Kawakami R, Guagliumi G, et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143(10):1031–1042. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

