Multi-omics approach dissects cis-regulatory mechanisms underlying North Carolina macular dystrophy, a retinal enhanceropathy
- PMID: 36243009
- PMCID: PMC9674966
- DOI: 10.1016/j.ajhg.2022.09.013
Multi-omics approach dissects cis-regulatory mechanisms underlying North Carolina macular dystrophy, a retinal enhanceropathy
Abstract
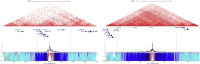
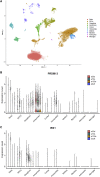
North Carolina macular dystrophy (NCMD) is a rare autosomal-dominant disease affecting macular development. The disease is caused by non-coding single-nucleotide variants (SNVs) in two hotspot regions near PRDM13 and by duplications in two distinct chromosomal loci, overlapping DNase I hypersensitive sites near either PRDM13 or IRX1. To unravel the mechanisms by which these variants cause disease, we first established a genome-wide multi-omics retinal database, RegRet. Integration of UMI-4C profiles we generated on adult human retina then allowed fine-mapping of the interactions of the PRDM13 and IRX1 promoters and the identification of eighteen candidate cis-regulatory elements (cCREs), the activity of which was investigated by luciferase and Xenopus enhancer assays. Next, luciferase assays showed that the non-coding SNVs located in the two hotspot regions of PRDM13 affect cCRE activity, including two NCMD-associated non-coding SNVs that we identified herein. Interestingly, the cCRE containing one of these SNVs was shown to interact with the PRDM13 promoter, demonstrated in vivo activity in Xenopus, and is active at the developmental stage when progenitor cells of the central retina exit mitosis, suggesting that this region is a PRDM13 enhancer. Finally, mining of single-cell transcriptional data of embryonic and adult retina revealed the highest expression of PRDM13 and IRX1 when amacrine cells start to synapse with retinal ganglion cells, supporting the hypothesis that altered PRDM13 or IRX1 expression impairs interactions between these cells during retinogenesis. Overall, this study provides insight into the cis-regulatory mechanisms of NCMD and supports that this condition is a retinal enhanceropathy.
Keywords: IRX1; North Carolina macular dystrophy, NCMD; PRDM13; UMI-4C; cis-regulatory elements, CREs; enhanceropathy; human retina; multi-omics; non-coding single-nucleotide variants, SNVs; whole-genome sequencing.
Crown Copyright © 2022. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures






References
-
- Farwell K.D., Shahmirzadi L., El-Khechen D., Powis Z., Chao E.C., Tippin Davis B., Baxter R.M., Zeng W., Mroske C., Parra M.C., et al. Enhanced utility of family-centered diagnostic exome sequencing with inheritance model-based analysis: Results from 500 unselected families with undiagnosed genetic conditions. Genet. Med. 2015;17:578–586. - PubMed
-
- Majewski J., Schwartzentruber J., Lalonde E., Montpetit A., Jabado N. What can exome sequencing do for you? J. Med. Genet. 2011;48:580–589. - PubMed
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

