Manipulation-specific cortical activity as mice handle food
- PMID: 36243014
- PMCID: PMC9691616
- DOI: 10.1016/j.cub.2022.09.045
Manipulation-specific cortical activity as mice handle food
Abstract
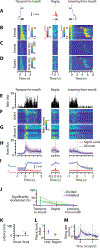
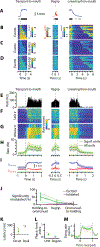
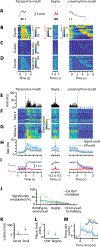
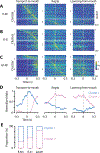
Food handling offers unique yet largely unexplored opportunities to investigate how cortical activity relates to forelimb movements in a natural, ethologically essential, and kinematically rich form of manual dexterity. To determine these relationships, we recorded high-speed (1,000 fps) video and multi-channel electrophysiological cortical spiking activity while mice handled food. The high temporal resolution of the video allowed us to decompose active manipulation ("oromanual") events into characteristic submovements, enabling event-aligned analysis of cortical activity. Activity in forelimb M1 was strongly modulated during food handling, generally higher during oromanual events and lower during holding intervals. Optogenetic silencing and stimulation of forelimb M1 neurons partially affected food-handling movements, exerting suppressive and activating effects, respectively. We also extended the analysis to forelimb S1 and lateral M1, finding broadly similar oromanual-related activity across all three areas. However, each area's activity displayed a distinct timing and phasic/tonic temporal profile, which was further analyzed by non-negative matrix factorization and demonstrated to be attributable to area-specific composition of activity classes. Current or future forelimb position could be accurately predicted from activity in all three regions, indicating that the cortical activity in these areas contains high information content about forelimb movements during food handling. These results thus establish that cortical activity during food handling is manipulation specific, distributed, and broadly similar across multiple sensorimotor areas while also exhibiting area- and submovement-specific relationships with the fast kinematic hallmarks of this natural form of complex free-object-handling manual dexterity.
Keywords: electrophysiology; food handling; forelimb; generalized linear model; manual dexterity; motor cortex; non-negative matrix factorization; optogenetics; oromanual; somatosensory cortex.
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures



References
-
- Svoboda K, and Li N (2018) Neural mechanisms of movement planning: motor cortex and beyond. Curr. Opin. Neurobiol. 49, 33–41. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

