Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: a first-in-patient, single-centre, single-arm, phase 1b trial
- PMID: 36243020
- PMCID: PMC9641975
- DOI: 10.1016/S1470-2045(22)00599-X
Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: a first-in-patient, single-centre, single-arm, phase 1b trial
Abstract
Background: Topotecan is cytotoxic to glioma cells but is clinically ineffective because of drug delivery limitations. Systemic delivery is limited by toxicity and insufficient brain penetrance, and, to date, convection-enhanced delivery (CED) has been restricted to a single treatment of restricted duration. To address this problem, we engineered a subcutaneously implanted catheter-pump system capable of repeated, chronic (prolonged, pulsatile) CED of topotecan into the brain and tested its safety and biological effects in patients with recurrent glioblastoma.
Methods: We did a single-centre, open-label, single-arm, phase 1b clinical trial at Columbia University Irving Medical Center (New York, NY, USA). Eligible patients were at least 18 years of age with solitary, histologically confirmed recurrent glioblastoma showing radiographic progression after surgery, radiotherapy, and chemotherapy, and a Karnofsky Performance Status of at least 70. Five patients had catheters stereotactically implanted into the glioma-infiltrated peritumoural brain and connected to subcutaneously implanted pumps that infused 146 μM topotecan 200 μL/h for 48 h, followed by a 5-7-day washout period before the next infusion, with four total infusions. After the fourth infusion, the pump was removed and the tumour was resected. The primary endpoint of the study was safety of the treatment regimen as defined by presence of serious adverse events. Analyses were done in all treated patients. The trial is closed, and is registered with ClinicalTrials.gov, NCT03154996.
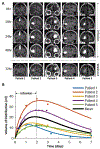
Findings: Between Jan 22, 2018, and July 8, 2019, chronic CED of topotecan was successfully completed safely in all five patients, and was well tolerated without substantial complications. The only grade 3 adverse event related to treatment was intraoperative supplemental motor area syndrome (one [20%] of five patients in the treatment group), and there were no grade 4 adverse events. Other serious adverse events were related to surgical resection and not the study treatment. Median follow-up was 12 months (IQR 10-17) from pump explant. Post-treatment tissue analysis showed that topotecan significantly reduced proliferating tumour cells in all five patients.
Interpretation: In this small patient cohort, we showed that chronic CED of topotecan is a potentially safe and active therapy for recurrent glioblastoma. Our analysis provided a unique tissue-based assessment of treatment response without the need for large patient numbers. This novel delivery of topotecan overcomes limitations in delivery and treatment response assessment for patients with glioblastoma and could be applicable for other anti-glioma drugs or other CNS diseases. Further studies are warranted to determine the effect of this drug delivery approach on clinical outcomes.
Funding: US National Institutes of Health, The William Rhodes and Louise Tilzer Rhodes Center for Glioblastoma, the Michael Weiner Glioblastoma Research Into Treatment Fund, the Gary and Yael Fegel Foundation, and The Khatib Foundation.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests JNB has a consulting agreement with Theracle and held the sponsor-investigator Investigational New Drug Application from the US Food and Drug Administration for this study. NYRA receives support from EMD Serono and Bruker Daltonics. FMI has obtained grants or contracts through Columbia from Merck, Bristol Myers Squibb, Roche, Sapience, Novocure, Celldex, Tocagen, Forma, Celldex, and Northwest Biotherapeutics; is in consulting agreements with Novocure, Regeneron, Tocagen, Alexion Pharmaceuticals, Abbvie, Guidepoint Global, Merck, Kiyatec, PPD, Massive Bio, Medtronic, MimiVax, Gennao Bio, and Xcures; has two US provisional patent applications (62/739,617 and 63/062,805) through Columbia University; received support for meetings and travel from Roche and Oncoceutics; and participates on advisory boards of Mimivax and Northwest Biotherapeutics. AMi is in consulting agreements and on the advisory board of Regeneron. PAS receives consulting fees from Wilson Sonsini and EpiCypher, received payment from AstraZeneca for an honorarium for a seminar, and royalties from Guardant Health through Harvard University. SZ is the paediatric oncology lead at Bristol Myers Squibb. ABL receives consulting fees or personal financial support for honoraria or meetings from Affinia, Bioclinica, Elsevier, Fondazion AIRC, National Cancer Institute, Novocure, Sapience, Leal, Abbott, AbbVie, Clinical Education Alliance, MJH Healthcare, Novartis, Northwest Biotherapeutics, Oligonation, Pfizer, Radiation Therapy Oncology Group Foundation, American Society of Clinical Oncology, Bayer, US Food and Drug Administration, Forma, Karyopharm, QED, Global Coalition for Adaptive Research, Matheson Foundation, NHS Blood and Transplant, SNO, and VBI Vaccines, and is on advisory boards of Abbvie, Bayer, Chimerix, Forma, Karyopharm, Novocure, Orbus, QED, and Vivacitas. No authors are employees of WHO, International Agency for Research on Cancer, or Pan American Health Organization. All other authors declare no competing interests.
Figures

Comment in
-
Chronic convection-enhanced intratumoural delivery of chemotherapy for glioblastoma.Lancet Oncol. 2022 Nov;23(11):1347-1348. doi: 10.1016/S1470-2045(22)00626-X. Epub 2022 Oct 13. Lancet Oncol. 2022. PMID: 36243021 Free PMC article. No abstract available.
References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. - PubMed
-
- Friedman HS, Kerby T, Fields S, Zilisch JE, Graden D, McLendon RE, et al. Topotecan treatment of adults with primary malignant glioma. The Brain Tumor Center at Duke. Cancer. 1999;85(5):1160–5. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

