Severe mammarenaviral disease in guinea pigs effectively treated by an orally bioavailable fusion inhibitor, alone or in combination with favipiravir
- PMID: 36243175
- PMCID: PMC10187609
- DOI: 10.1016/j.antiviral.2022.105444
Severe mammarenaviral disease in guinea pigs effectively treated by an orally bioavailable fusion inhibitor, alone or in combination with favipiravir
Abstract
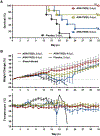
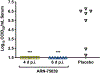
Infections by pathogenic New World mammarenaviruses (NWM)s, including Junín virus (JUNV), can result in a severe life-threatening viral hemorrhagic fever syndrome. In the absence of FDA-licensed vaccines or antivirals, these viruses are considered high priority pathogens. The mammarenavirus envelope glycoprotein complex (GPC) mediates pH-dependent fusion between viral and cellular membranes, which is essential to viral entry and may be vulnerable to small-molecule inhibitors that disrupt this process. ARN-75039 is a potent fusion inhibitor of a broad spectrum of pseudotyped and native mammarenaviruses in cell culture and Tacaribe virus infection in mice. In the present study, we evaluated ARN-75039 against pathogenic JUNV in the rigorous guinea pig infection model. The compound was well-tolerated and had favorable pharmacokinetics supporting once-per-day oral dosing in guinea pigs. Importantly, significant protection against JUNV challenge was observed even when ARN-75039 was withheld until 6 days after the viral challenge when clinical signs of disease are starting to develop. We also show that ARN-75039 combination treatment with favipiravir, a viral polymerase inhibitor, results in synergistic activity in vitro and improves survival outcomes in JUNV-challenged guinea pigs. Our findings support the continued development of ARN-75039 as an attractive therapeutic candidate for treating mammarenaviral hemorrhagic fevers, including those associated with NWM infection.
Keywords: ARN-75039; Antiviral; Arenavirus; Favipiravir; Junin virus; Mammarenavirus.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Arisan Therapeutics has commercial interests and has filed multiple patent applications on ARN-75039, for which K.M., V.R.G., and G.H. are co-inventors. The other authors declare no competing interests.
Figures






Similar articles
-
Coadministration of LHF-535 and favipiravir protects against experimental Junín virus infection and disease.Antiviral Res. 2024 Sep;229:105952. doi: 10.1016/j.antiviral.2024.105952. Epub 2024 Jun 28. Antiviral Res. 2024. PMID: 38945484 Free PMC article.
-
Favipiravir (T-705) inhibits Junín virus infection and reduces mortality in a guinea pig model of Argentine hemorrhagic fever.PLoS Negl Trop Dis. 2013 Dec 26;7(12):e2614. doi: 10.1371/journal.pntd.0002614. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24386500 Free PMC article.
-
Guinea Pig Transferrin Receptor 1 Mediates Cellular Entry of Junín Virus and Other Pathogenic New World Arenaviruses.J Virol. 2020 Jan 31;94(4):e01278-19. doi: 10.1128/JVI.01278-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31748396 Free PMC article.
-
The Virus-Host Interplay in Junín Mammarenavirus Infection.Viruses. 2022 May 24;14(6):1134. doi: 10.3390/v14061134. Viruses. 2022. PMID: 35746604 Free PMC article. Review.
-
Favipiravir (T-705), a novel viral RNA polymerase inhibitor.Antiviral Res. 2013 Nov;100(2):446-54. doi: 10.1016/j.antiviral.2013.09.015. Epub 2013 Sep 29. Antiviral Res. 2013. PMID: 24084488 Free PMC article. Review.
Cited by
-
Delayed low-dose oral administration of 4'-fluorouridine inhibits pathogenic arenaviruses in animal models of lethal disease.Sci Transl Med. 2024 Nov 20;16(774):eado7034. doi: 10.1126/scitranslmed.ado7034. Epub 2024 Nov 20. Sci Transl Med. 2024. PMID: 39565871
-
Entry inhibitors as arenavirus antivirals.Front Microbiol. 2024 Apr 8;15:1382953. doi: 10.3389/fmicb.2024.1382953. eCollection 2024. Front Microbiol. 2024. PMID: 38650890 Free PMC article. Review.
-
Coadministration of LHF-535 and favipiravir protects against experimental Junín virus infection and disease.Antiviral Res. 2024 Sep;229:105952. doi: 10.1016/j.antiviral.2024.105952. Epub 2024 Jun 28. Antiviral Res. 2024. PMID: 38945484 Free PMC article.
-
Treatment of highly virulent mammarenavirus infections-status quo and future directions.Expert Opin Drug Discov. 2024 May;19(5):537-551. doi: 10.1080/17460441.2024.2340494. Epub 2024 Apr 12. Expert Opin Drug Discov. 2024. PMID: 38606475 Free PMC article. Review.
References
-
- Briese T, Paweska JT, McMullan LK, Hutchison SK, Street C, Palacios G, Khristova ML, Weyer J, Swanepoel R, Egholm M, Nichol ST, Lipkin WI, 2009. Genetic detection and characterization of Lujo virus, a new hemorrhagic fever-associated arenavirus from southern Africa. PLoS Pathog. 5, e1000455. - PMC - PubMed
-
- Delang L, Abdelnabi R, Neyts J, 2018. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antivir. Res. 153, 85–94. - PubMed
-
- Emonet SF, Seregin AV, Yun NE, Poussard AL, Walker AG, de la Torre JC, Paessler S, 2011. Rescue from cloned cDNAs and in vivo characterization of recombinant pathogenic Romero and live-attenuated Candid #1 strains of Junin virus, the causative agent of Argentine hemorrhagic fever disease. J. Virol. 85, 1473–1483. - PMC - PubMed
-
- Enria DA, Ambrosio AM, Briggiler AM, Feuillade MR, Crivelli E, 2010. [Candid#1 vaccine against Argentine hemorrhagic fever produced in Argentina. Immunogenicity and safety]. Medicina 70, 215–222. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

