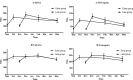
Intra-season waning of immunity following the seasonal influenza vaccine in early and late vaccine recipients
- PMID: 36243199
- PMCID: PMC9556766
- DOI: 10.1016/j.jinf.2022.10.011
Intra-season waning of immunity following the seasonal influenza vaccine in early and late vaccine recipients
Keywords: Health care workers; Inactivated influenza vaccine; Waning immunity.
Conflict of interest statement
Declaration of Competing Interest SGS declares an NIH grant, OptumLabs research credits, participation in Advisory Boards for influenza vaccines for Seqiris and Sanofi (no remuneration received), member of the WHO Strategic Advisory Group of Experts (SAGE) on Immunization Working Group on Influenza, and invited member of the National Influenza Surveillance Committee. IB declares shares in an influenza vaccine manufacturing company. MP declares research grants paid to institution from UKRI-MRC, NIHR and Gilead Sciences, and consulting fees from QIAGEN.
Figures
Comment on
-
Decline in antibody responses to SARS-CoV-2 post-vaccination poses a risk to health care workers.J Infect. 2022 Sep;85(3):334-363. doi: 10.1016/j.jinf.2022.06.008. Epub 2022 Jun 12. J Infect. 2022. PMID: 35705136 Free PMC article. No abstract available.
References
-
- Kissling E., Valenciano M., Larrauri A., Oroszi B., Cohen J.M., Nunes B., et al. Low and decreasing vaccine effectiveness against influenza A(H3) in 2011/12 among vaccination target groups in Europe: results from the I-MOVE multicentre case–control study. Eurosurveillance. 2013;18(5):20390. - PubMed
-
- World Health Organization = Organisation mondiale de la S Weekly Epidemiological Record, 2020, vol. 95, 12 [full issue] Weekly Epidemiological Record. 2020;95(12):105–116.
-
- World Health O . World Health Organization; Geneva: 2011. Manual for the laboratory diagnosis and virological surveillance of influenza.
-
- (CPMP) ECfPMP . The European Agency for the Evaluation of Medicinal Products; London, UK: 1997. Note for guidance on harmonisation of requirements for influenza vaccines.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical