Lcn2 mediates adipocyte-muscle-tumor communication and hypothermia in pancreatic cancer cachexia
- PMID: 36243318
- PMCID: PMC9596731
- DOI: 10.1016/j.molmet.2022.101612
Lcn2 mediates adipocyte-muscle-tumor communication and hypothermia in pancreatic cancer cachexia
Abstract
Objective: Adipose tissue is the largest endocrine organ. When activated by cancer cells, adipocytes secrete adipocytokines and release fatty acids, which are then transferred to cancer cells and used for structural and biochemical support. How this metabolic symbiosis between cancer cells and adipocytes affects skeletal muscle and thermogenesis during cancer cachexia is unknown. Cancer cachexia is a multiorgan syndrome and how the communication between tissues is established has yet to be determined. We investigated adipose tissue secretory factors and explored their role in crosstalk of adipocytes, muscle, and tumor during pancreatic cancer cachexia.
Methods: We used a pancreatic cancer cachexia mouse model generated by syngenic implantation of pancreatic ductal adenocarcinoma (PDAC) cells (KPC) intraperitoneally into C57BL/6 mice and Lcn2-knockout mice. For in vitro studies, adipocytes (3T3-L1 and primary adipocytes), cachectic cancer cells (Panc0203), non-cachectic cancer cells (Du145 cells), and skeletal muscle cells (C2C12 myoblasts) were used.
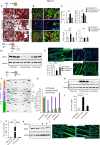
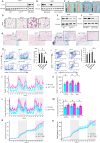
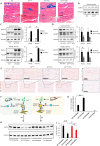
Results: To identify molecules involved in the crosstalk of adipose tissue with muscle and tumors, we treated 3T3-L1 adipocytes with conditioned medium (CM) from cancer cells. Upon screening the secretomes from PDAC-induced adipocytes, several adipocytokines were identified, including lipocalin 2 (Lcn2). We investigated Lcn2 as a potential mediator of cachexia induced by adipocytes in response to PDAC. During tumor progression, mice exhibited a decline in body weight gain, which was accompanied by loss of adipose and muscle tissues. Tumor-harboring mice developed drastic hypothermia because of a dramatic loss of fat in brown adipose tissue (BAT) and suppression of the thermogenesis pathway. We inhibited Lcn2 with an anti-Lcn2 antibody neutralization or genomic ablation in mice. Lcn2 deficiency significantly improved body temperature in tumor-bearing mice, which was supported by the increased expression of Ucp1 and β3-adrenergic receptor in BAT. In addition, Lcn2 inhibition abrogated the loss of fat and muscle in tumor-bearing mice. In contrast to tumor-bearing WT mice, the corresponding Lcn2-knockout mice showed reduced ATGL expression in iWAT and decreased the expression of muscle atrophy molecular markers MuRF-1 and Fbx32.
Conclusions: This study showed that Lcn2 is causally involved in the dysregulation of adipose tissue-muscle-tumor crosstalk during pancreatic cancer cachexia. Therapeutic targets that suppress Lcn2 may minimize the progression of cachexia.
Keywords: BAT; Cachexia; Hypothermia; Lcn2; PDAC; Thermogenesis.
Published by Elsevier GmbH.
Figures



References
-
- Society A.C. American Cancer Society; 2022. Cancer facts & figures.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

