SAP deficiency aggravates periodontitis possibly via C5a-C5aR signaling-mediated defective macrophage phagocytosis of Porphyromonas gingivalis
- PMID: 36243399
- PMCID: PMC10403661
- DOI: 10.1016/j.jare.2022.10.003
SAP deficiency aggravates periodontitis possibly via C5a-C5aR signaling-mediated defective macrophage phagocytosis of Porphyromonas gingivalis
Abstract
Introduction: Serum amyloid P component (SAP) regulates the innate immune system and microbial diseases. Periodontitis is an inflammatory oral disease developed by the host immune system's interaction with the dysbiotic oral microbiome, thereby SAP could play a role in periodontitis pathogenicity.
Objectives: To investigate the role of SAP in oral microbiome modulation and peridontitis pathogenicity.
Methods: In this study, wildtype and SAP-knockout (KO) mice were used. Ligature-based periodontitis was developed in mice. Oral microbiome diversity was analyzed by 16 s rRNA sequencing. Macrophages and Porphyromonas gingivalis (P. gingivalis) co-culture system analyzed the effect of SAP in macrophage phagocytosis of P. gingivalis.
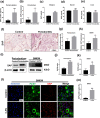
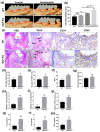
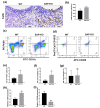
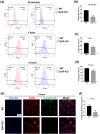
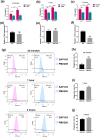
Results: The level of SAP was upregulated in the periodontitis-affected periodontium of humans and mice but not in the liver and blood circulation. Periodontal macrophages were the key source of upregulated SAP in periodontitis. SAP-KO aggravated periodontal inflammation, periodontitis, and a higher number of M1-type inflammatory macrophage infiltration in the periodontium. The oral microbiome of SAP-KO periodontitis mice was altered with a higher abundance of Porphyromonas at the genus level. SAP-KO macrophages showed compromised phagocytosis of P. gingivalis in the co-culture system. Co-culture of SAP-KO macrophages and P. gingivalis induced the C5a expression and exogenous SAP treatment nullified this effect. Exogenous recombinant SAP treatment did not affect P. gingivalis growth and opsonization. PMX205, an antagonist of C5a, treatment robustly enhanced P. gingivalis phagocytosis by SAP-KO macrophages, indicating the involvement of the C5a-C5aR signaling in the compromised P. gingivalis phagocytosis by SAP-KO macrophages.
Conclusion: SAP deficiency aggravates periodontitis possibly via C5a-C5aR signaling-mediated defective macrophage phagocytosis of P. gingivalis. A higher abundance of P. gingivalis during SAP deficiency could promote M1 macrophage polarization and periodontitis. This finding suggests the possible protecting role of elevated levels of periodontal SAP against periodontitis progression.
Keywords: C5a-C5aR signaling; Macrophages; Periodontitis; Porphyromonas gingivalis; Serum amyloid P component.
Copyright © 2023. Production and hosting by Elsevier B.V.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Anti-inflammatory Effects of PMX205 in Mouse Macrophage Periodontitis Model.Iran J Immunol. 2018 Jun;15(2):84-96. Iran J Immunol. 2018. PMID: 29947338
-
The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss.J Immunol. 2011 Jan 15;186(2):869-77. doi: 10.4049/jimmunol.1003252. Epub 2010 Dec 13. J Immunol. 2011. PMID: 21149611 Free PMC article.
-
Sialidase facilitates Porphyromonas gingivalis immune evasion by reducing M1 polarization, antigen presentation, and phagocytosis of infected macrophages.Front Cell Infect Microbiol. 2023 May 30;13:1173899. doi: 10.3389/fcimb.2023.1173899. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37325520 Free PMC article.
-
Porphyromonas Gingivalis in the Development of Periodontitis: Impact on Dysbiosis and Inflammation.Arch Razi Inst. 2022 Oct 31;77(5):1539-1551. doi: 10.22092/ARI.2021.356596.1875. eCollection 2022 Oct. Arch Razi Inst. 2022. PMID: 37123122 Free PMC article. Review.
-
Effects of Porphyromonas gingivalis Outer Membrane Vesicles (OMVs) on Macrophages in Periodontitis.Oral Dis. 2025 Jul;31(7):1973-1981. doi: 10.1111/odi.15264. Epub 2025 Jan 30. Oral Dis. 2025. PMID: 39887837 Review.
Cited by
-
The nano-artificial periosteum made of PCL/MgO/AS-IV enhances MC3T3-E1 cell osteogenic differentiation and promotes bone defect repair via the EphB4/EphrinB2 signaling pathway.Heliyon. 2024 May 28;10(11):e32036. doi: 10.1016/j.heliyon.2024.e32036. eCollection 2024 Jun 15. Heliyon. 2024. PMID: 38882277 Free PMC article.
-
A Bibliometric Analysis of Macrophage Research Associated with Periodontitis Over the Past Two Decades.Int Dent J. 2025 Feb;75(1):363-374. doi: 10.1016/j.identj.2024.11.016. Epub 2024 Dec 12. Int Dent J. 2025. PMID: 39672778 Free PMC article.
-
Oral Microbiome and Alzheimer's Disease.Microorganisms. 2023 Oct 13;11(10):2550. doi: 10.3390/microorganisms11102550. Microorganisms. 2023. PMID: 37894208 Free PMC article.
References
-
- Matsha T.E., Prince Y., Davids S., Chikte U., Erasmus R.T., Kengne A.P., et al. Oral microbiome signatures in diabetes mellitus and periodontal disease. J Dent Res. 2020;99(6):658–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

