Emergence of BRCA Reversion Mutations in Patients with Metastatic Castration-resistant Prostate Cancer After Treatment with Rucaparib
- PMID: 36243543
- PMCID: PMC10398818
- DOI: 10.1016/j.eururo.2022.09.010
Emergence of BRCA Reversion Mutations in Patients with Metastatic Castration-resistant Prostate Cancer After Treatment with Rucaparib
Abstract
Background: Poly(adenosine diphosphate-ribose) polymerase (PARP) inhibitors are approved in the USA for the treatment of patients with BRCA1 or BRCA2 (BRCA) mutated (BRCA+) metastatic castration-resistant prostate cancer (mCRPC). BRCA reversion mutations are a known mechanism of acquired resistance to PARP inhibitors in multiple cancer types, although their impact and prevalence in mCRPC remain unknown.
Objective: To examine the prevalence of BRCA reversion mutations in the plasma of patients with BRCA+ mCRPC after progression on rucaparib.
Design, setting, and participants: Men with BRCA+ mCRPC enrolled in Trial of Rucaparib in Prostate Indications 2 (TRITON2) were treated with rucaparib after progressing on one to two lines of androgen receptor-directed and one taxane-based therapy. Cell-free DNA from the plasma of 100 patients, collected at the end of treatment after confirmed progression before May 5, 2020, was queried for BRCA reversion mutations using next-generation sequencing (NGS).
Outcome measurements and statistical analysis: The association of clinical efficacy and postprogression genomics was measured in 100 patients with BRCA+ mCRPC treated with rucaparib.
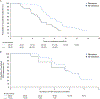
Results and limitations: No baseline BRCA reversion mutations were observed in 100 BRCA+ patients. NGS identified somatic BRCA reversion mutations in 39% (39/100) of patients after progression. Reversion rates were similar for BRCA2 and BRCA1, irrespective of germline or somatic status, but higher in samples with a high tumor DNA fraction. Most patients with reversions (74%, 29/39) had two or more reversion mutations occurring subclonally at lower allele frequencies than the original BRCA mutations. The incidence of BRCA reversion mutations increased with the duration of rucaparib treatment. The frequency of reversion mutations was higher in patients with an objective (58%) or a prostate-specific antigen (69%) response compared with those without either (39% and 29%, respectively).
Conclusions: These findings suggest that BRCA reversion mutations are a significant mechanism of acquired resistance to rucaparib in patients with BRCA+ mCRPC, with evidence of subclonal convergence promoting systemic resistance.
Patient summary: Men with BRCA mutated metastatic castration-resistant prostate cancer enrolled in TRITON2 were treated with rucaparib after progressing on one to two lines of androgen receptor-directed and one taxane-based therapy. Cell-free DNA from the plasma of 100 patients, collected after radiographic or prostate-specific antigen progression before May 5, 2020, was analyzed by next-generation sequencing and queried for BRCA reversion mutations.
Keywords: Acquired resistance; BRCA reversion mutations; Circulating tumor DNA; Metastatic castration-resistant prostate cancer; Next-generation sequencing; Poly(adenosine diphosphate-ribose) polymerase inhibitor.
Copyright © 2022 European Association of Urology. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Figures
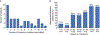

Comment in
-
BRCA Reversion Mutations in Metastatic Castration-Resistant Prostate Cancer.Eur Urol. 2023 Mar;83(3):210-211. doi: 10.1016/j.eururo.2022.09.031. Epub 2022 Oct 20. Eur Urol. 2023. PMID: 36273936 No abstract available.
-
Re: Andrea Loehr, Arif Hussain, Akash Patnaik, et al. Emergence of BRCA Reversion Mutations in Patients with Metastatic Castration-resistant Prostate Cancer After Treatment with Rucaparib. Eur Urol. In press. https://doi.org/10.1016/j.eururo.2022.09.010.Eur Urol. 2023 Mar;83(3):e76-e77. doi: 10.1016/j.eururo.2022.12.024. Epub 2023 Jan 3. Eur Urol. 2023. PMID: 36604272 No abstract available.
-
Reply to Feng Qi, Zicheng Xu, and Qing Zou's Letter to the Editor re: Andrea Loehr, Arif Hussain, Akash Patnaik, et al. Emergence of BRCA Reversion Mutations in Patients with Metastatic Castration-resistant Prostate Cancer After Treatment with Rucaparib. Eur Urol. In press. https://doi.org/10.1016/j.eururo.2022.09.010.Eur Urol. 2023 Mar;83(3):e78. doi: 10.1016/j.eururo.2022.12.023. Epub 2023 Jan 7. Eur Urol. 2023. PMID: 36623951 No abstract available.
Similar articles
-
Rucaparib for the Treatment of Metastatic Castration-resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study.Eur Urol. 2023 Sep;84(3):321-330. doi: 10.1016/j.eururo.2023.05.021. Epub 2023 Jun 3. Eur Urol. 2023. PMID: 37277275 Free PMC article. Clinical Trial.
-
Response to Rucaparib in BRCA-Mutant Metastatic Castration-Resistant Prostate Cancer Identified by Genomic Testing in the TRITON2 Study.Clin Cancer Res. 2021 Dec 15;27(24):6677-6686. doi: 10.1158/1078-0432.CCR-21-2199. Epub 2021 Oct 1. Clin Cancer Res. 2021. PMID: 34598946 Free PMC article.
-
Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration.J Clin Oncol. 2020 Nov 10;38(32):3763-3772. doi: 10.1200/JCO.20.01035. Epub 2020 Aug 14. J Clin Oncol. 2020. PMID: 32795228 Free PMC article.
-
When and How to Use PARP Inhibitors in Prostate Cancer: A Systematic Review of the Literature with an Update on On-Going Trials.Eur Urol Oncol. 2020 Oct;3(5):594-611. doi: 10.1016/j.euo.2020.07.005. Epub 2020 Aug 17. Eur Urol Oncol. 2020. PMID: 32814685
-
A Novel Use of Olaparib for the Treatment of Metastatic Castration-Recurrent Prostate Cancer.Pharmacotherapy. 2017 Nov;37(11):1406-1414. doi: 10.1002/phar.2027. Pharmacotherapy. 2017. PMID: 28895177 Review.
Cited by
-
PARP inhibitors in prostate cancer: clinical applications.Mol Biol Rep. 2024 Oct 30;51(1):1103. doi: 10.1007/s11033-024-10034-5. Mol Biol Rep. 2024. PMID: 39476131 Review.
-
Hypoxia-inducible factor 1A inhibition overcomes castration resistance of prostate tumors.EMBO Mol Med. 2023 Jun 7;15(6):e17209. doi: 10.15252/emmm.202217209. Epub 2023 Apr 18. EMBO Mol Med. 2023. PMID: 37070472 Free PMC article.
-
Unravelling the molecular basis of PARP inhibitor resistance in prostate cancer with homologous recombination repair deficiency.Int Rev Cell Mol Biol. 2024;389:257-301. doi: 10.1016/bs.ircmb.2024.03.004. Epub 2024 Mar 31. Int Rev Cell Mol Biol. 2024. PMID: 39396849 Free PMC article. Review.
-
Toward Informed Selection and Interpretation of Clinical Genomic Tests in Prostate Cancer.JCO Precis Oncol. 2024 Mar;8:e2300654. doi: 10.1200/PO.23.00654. JCO Precis Oncol. 2024. PMID: 38547422 Free PMC article.
-
Omics-Mediated Treatment for Advanced Prostate Cancer: Moving Towards Precision Oncology.Int J Mol Sci. 2025 Aug 2;26(15):7475. doi: 10.3390/ijms26157475. Int J Mol Sci. 2025. PMID: 40806607 Free PMC article. Review.
References
-
- Green F, Shaprio JD, McDermott R, et al. Comprehensive genomic profiling of >1000 plasma and tumor tissue samples from metastatic castration-resistant prostate cancer (mCRPC) patients gives insight into targeted treatment strategies. Cancer Res 2019;79(suppl 13):727.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

