Tranexamic acid dose-response relationship for antifibrinolysis in postpartum haemorrhage during Caesarean delivery: TRACES, a double-blind, placebo-controlled, multicentre, dose-ranging biomarker study
- PMID: 36243576
- PMCID: PMC9748994
- DOI: 10.1016/j.bja.2022.08.033
Tranexamic acid dose-response relationship for antifibrinolysis in postpartum haemorrhage during Caesarean delivery: TRACES, a double-blind, placebo-controlled, multicentre, dose-ranging biomarker study
Abstract
Background: The optimal dose of tranexamic acid to inhibit hyperfibrinolysis in postpartum haemorrhage is unclear. Tranexamic Acid to Reduce Blood Loss in Hemorrhagic Cesarean Delivery (TRACES) was a double-blind, placebo-controlled, randomised, multicentre dose-ranging study to determine the dose-effect relationship for two regimens of intravenous tranexamic acid vs placebo.
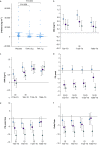
Methods: Women experiencing postpartum haemorrhage during Caesarean delivery were randomised to receive placebo (n=60), tranexamic acid 0.5 g (n=57), or tranexamic acid 1 g i.v. (n=58). Biomarkers of fibrinolytic activation were assayed at five time points, with inhibition of hyperfibrinolysis defined as reductions in the increase over baseline in D-dimer and plasmin-antiplasmin levels and in the plasmin peak time.
Results: In the placebo group, hyperfibrinolysis was evidenced by a mean increase over baseline [95% confidence interval] of 93% [68-118] for D-dimer level at 120 min and 56% [25-87] for the plasmin-antiplasmin level at 30 min. A dose of tranexamic acid 1 g was associated with smaller increases over baseline (D-dimers: 38% [13-63] [P=0.003 vs placebo]; plasmin-antiplasmin: -2% [-32 to 28] [P=0.009 vs placebo]). A dose of tranexamic acid 0.5 g was less potent, with non-significant reductions (D-dimers: 58% [32-84] [P=0.06 vs placebo]; plasmin-antiplasmin: 13% [18-43] [P=0.051]). Although both tranexamic acid doses reduced the plasmin peak, reduction in plasmin peak time was significant only for the 1 g dose of tranexamic acid.
Conclusions: Fibrinolytic activation was significantly inhibited by a dose of intravenous tranexamic acid 1 g but not 0.5 g. Pharmacokinetic-pharmacodynamic modelling of these data might identify the best pharmacodynamic monitoring criteria and the optimal tranexamic acid dosing regimen for treatment of postpartum haemorrhage.
Clinical trial registration: NCT02797119.
Keywords: D-dimer; antifibrinolytic drug; fibrinogen; fibrinolysis; plasmin; plasmin–antiplasmin complex; postpartum haemorrhage; thrombin; tranexamic acid.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures
References
-
- Ducloy-Bouthors A.S., Duhamel A., Kipnis E., et al. Postpartum haemorrhage related early increase in D-dimers is inhibited by tranexamic acid: haemostasis parameters of a randomized controlled open labelled trial. Br J Anaesth. 2016;116:641–648. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

