Pan-cancer analysis of oncogenic TNFAIP2 identifying its prognostic value and immunological function in acute myeloid leukemia
- PMID: 36243694
- PMCID: PMC9571470
- DOI: 10.1186/s12885-022-10155-9
Pan-cancer analysis of oncogenic TNFAIP2 identifying its prognostic value and immunological function in acute myeloid leukemia
Abstract
Background: Tumor necrosis factor alpha-induced protein 2 (TNFAIP2), a TNFα-inducible gene, appears to participate in inflammation, immune response, hematopoiesis, and carcinogenesis. However, the potential role of TNFAIP2 in the development of acute myeloid leukemia (AML) remains unknow yet. Therefore, we aimed to study the biological role of TNFAIP2 in leukemogenesis.
Methods: TNFAIP2 mRNA level, prognostic value, co-expressed genes, differentially expressed genes, DNA methylation, and functional enrichment analysis in AML patients were explored via multiple public databases, including UALCAN, GTEx portal, Timer 2.0, LinkedOmics, SMART, MethSurv, Metascape, GSEA and String databases. Data from The Cancer Genome Atlas (TCGA), Gene Expression Omnibus (GEO) and Beat AML database were used to determine the associations between TNFAIP2 expression and various clinical or genetic parameters of AML patients. Moreover, the biological functions of TNFAIP2 in AML were investigated through in vitro experiments.
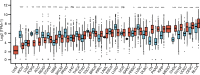
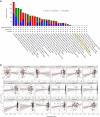
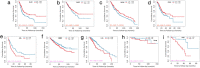
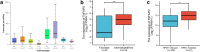
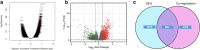
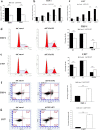
Results: By large-scale data mining, our study indicated that TNFAIP2 was differentially expressed across different normal and tumor tissues. TNFAIP2 expression was significantly increased in AML, particularly in French-American-British (FAB) classification M4/M5 patients, compared with corresponding control tissues. Overexpression of TNFAIP2 was an independent poor prognostic factor of overall survival (OS) and was associated with unfavorable cytogenetic risk and gene mutations in AML patients. DNA hypermethylation of TNFAIP2 at gene body linked to upregulation of TNFAIP2 and inferior OS in AML. Functional enrichment analysis indicated immunomodulation function and inflammation response of TNFAIP2 in leukemogenesis. Finally, the suppression of TNFAIP resulted in inhibition of proliferation by altering cell-cycle progression and increase of cell death by promoting early and late apoptosis in THP-1 and U937AML cells.
Conclusion: Collectively, the oncogenic TNFAIP2 can function as a novel biomarker and prognostic factor in AML patients. The immunoregulation function of TNFAIP2 warrants further validation in AML.
Keywords: Acute myeloid leukemia; Immunomodulation; Oncogenic; Prognosis; TNFAIP2.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
-
- Juliusson G. Older patients with acute myeloid leukemia benefit from intensive chemotherapy: an update from the Swedish acute leukemia registry. Clin Lymphoma Myeloma Leuk. 2011;11(Suppl 1):S9–54. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

