Data standards for atrial fibrillation/flutter and catheter ablation: the European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart)
- PMID: 36243903
- PMCID: PMC10495697
- DOI: 10.1093/ehjqcco/qcac068
Data standards for atrial fibrillation/flutter and catheter ablation: the European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart)
Abstract
Aims: Standardized data definitions are essential for monitoring and assessment of care and outcomes in observational studies and randomized controlled trials (RCTs). The European Unified Registries for Heart Care Evaluation and Randomized Trials (EuroHeart) project of the European Society of Cardiology aimed to develop contemporary data standards for atrial fibrillation/flutter (AF/AFL) and catheter ablation.
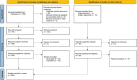
Methods and results: We used the EuroHeart methodology for the development of data standards and formed a Working Group comprising 23 experts in AF/AFL and catheter ablation registries, as well as representatives from the European Heart Rhythm Association and EuroHeart. We conducted a systematic literature review of AF/AFL and catheter ablation registries and data standard documents to generate candidate variables. We used a modified Delphi method to reach a consensus on a final variable set. For each variable, the Working Group developed permissible values and definitions, and agreed as to whether the variable was mandatory (Level 1) or additional (Level 2). In total, 70 Level 1 and 92 Level 2 variables were selected and reviewed by a wider Reference Group of 42 experts from 24 countries. The Level 1 variables were implemented into the EuroHeart IT platform as the basis for continuous registration of individual patient data.
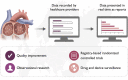
Conclusion: By means of a structured process and working with international stakeholders, harmonized data standards for AF/AFL and catheter ablation for AF/AFL were developed. In the context of the EuroHeart project, this will facilitate country-level quality of care improvement, international observational research, registry-based RCTs, and post-marketing surveillance of devices and pharmacotherapies.
Keywords: Atrial fibrillation; Atrial flutter; Catheter ablation; Data standards; Data variables; EuroHeart.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
G.B. reports, outside the submitted work, institutional research grants from Pfizer, expert committee and consulting fees to his institution from Bayer. Honoraria for lectures and scientific advice from AstraZeneca, Boehringer Ingelheim, Novo Nordisk, Pfizer, and Sanofi.
Figures

References
-
- Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist Cet al. ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European. Eur Heart J 2021;42:373–498. - PubMed
-
- Rahman F, Kwan GF, Benjamin EJ.. Global epidemiology of atrial fibrillation. Nat Rev Cardiol 2014;11:639–654. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

