Fluid shear stress promotes periodontal ligament cells proliferation via p38-AMOT-YAP
- PMID: 36244032
- PMCID: PMC11802950
- DOI: 10.1007/s00018-022-04591-w
Fluid shear stress promotes periodontal ligament cells proliferation via p38-AMOT-YAP
Abstract
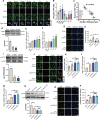
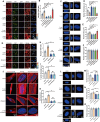
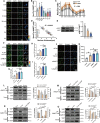
Periodontal ligament (PDL) cells are a promising tool for periodontal regeneration therapy. Achieving a sufficient number of PDL cells is essential to PDL regeneration. In our study, appropriate flow shear stress (FSS, 1-6 dyn/cm2) promotes the proliferation of PDL cells. FSS remodels cytoskeleton and focal adhesion in a duration-dependent manner. FSS induces PDL cells to form the actin cap within 10 min, flattens the nuclei, and increases the nuclear pore size, which promotes nuclear translocation of Yes-associated protein (YAP). FSS activates p38, which plays a dual function in YAP regulation. p38 regulates the phosphorylation of Akt and cofilin, as well as induced F-actin polymerization to induce YAP activity. In addition, p38 inhibits pLATS and consecutively regulates angiomotin (AMOT) and YAP phosphorylation. AMOT competitively binds to F-actin and YAP to participate in FSS-mediated YAP nuclear translocation and cell proliferation. Taken collectively, our results provide mechanistic insights into the role of p38-AMOT-YAP in FSS-mediated PDL cells proliferation and indicate potential applications in dental regenerative medicine.
Keywords: Biomechanics; LINC; Mechanotransduction; Nuclear changes; Nuclear-cytoplasmic translocation.
© 2022. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that there are no conflicts of interest regarding the material discussed in the manuscript.
Figures




References
-
- Pihlstrom BL, Michalowicz BS, Johnson NW (2005) Periodontal diseases. Lancet 366:1809–1820. 10.1016/S0140-6736(05)67728-8 - PubMed
-
- Kinane DF, Stathopoulou PG, Papapanou PN (2017) Periodontal diseases. Nat Rev Dis Primers 22:17038–17049. 10.1038/nrdp.2017.38 - PubMed
-
- Chen FM, Sun HH, Lu H, Yu Q (2012) Stem cell-delivery therapeutics for periodontal tissue regeneration. Biomaterials 33:6320–6344. 10.1016/j.biomaterials.2012.05.048 - PubMed
-
- Iwata T, Yamato M, Zhang Z et al (2010) Validation of human periodontal ligament-derived cells as a reliable source for cytotherapeutic use. J Clin Periodontol 37:1088–1099. 10.1111/j.1600-051X.2010.01597.x - PubMed
-
- Nakdilok K, Langsa-ard S, Krisanaprakornkit S et al (2020) Enhancement of human periodontal ligament by preapplication of orthodontic loading. Am J Orthod Dentofacial Orthop 157:186–193. 10.1016/j.ajodo.2019.03.019 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

