Traumatic axonopathy in spinal tracts after impact acceleration head injury: Ultrastructural observations and evidence of SARM1-dependent axonal degeneration
- PMID: 36244414
- PMCID: PMC10321775
- DOI: 10.1016/j.expneurol.2022.114252
Traumatic axonopathy in spinal tracts after impact acceleration head injury: Ultrastructural observations and evidence of SARM1-dependent axonal degeneration
Abstract
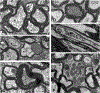
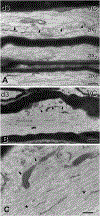
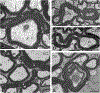
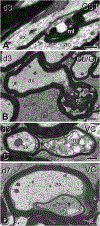
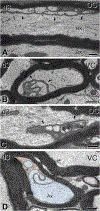
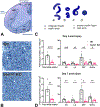
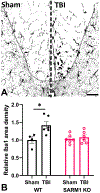
Traumatic axonal injury (TAI) and the associated axonopathy are common consequences of traumatic brain injury (TBI) and contribute to significant neurological morbidity. It has been previously suggested that TAI activates a highly conserved program of axonal self-destruction known as Wallerian degeneration (WD). In the present study, we utilize our well-established impact acceleration model of TBI (IA-TBI) to characterize the pathology of injured myelinated axons in the white matter tracks traversing the ventral, lateral, and dorsal spinal columns in the mouse and assess the effect of Sterile Alpha and TIR Motif Containing 1 (Sarm1) gene knockout on acute and subacute axonal degeneration and myelin pathology. In silver-stained preparations, we found that IA-TBI results in white matter pathology as well as terminal field degeneration across the rostrocaudal axis of the spinal cord. At the ultrastructural level, we found that traumatic axonopathy is associated with diverse types of axonal and myelin pathology, ranging from focal axoskeletal perturbations and focal disruption of the myelin sheath to axonal fragmentation. Several morphological features such as neurofilament compaction, accumulation of organelles and inclusions, axoskeletal flocculation, myelin degeneration and formation of ovoids are similar to profiles encountered in classical examples of WD. Other profiles such as excess myelin figures and inner tongue evaginations are more typical of chronic neuropathies. Stereological analysis of pathological axonal and myelin profiles in the ventral, lateral, and dorsal columns of the lower cervical cord (C6) segments from wild type and Sarm1 KO mice at 3 and 7 days post IA-TBI (n = 32) revealed an up to 90% reduction in the density of pathological profiles in Sarm1 KO mice after IA-TBI. Protection was evident across all white matter tracts assessed, but showed some variability. Finally, Sarm1 deletion ameliorated the activation of microglia associated with TAI. Our findings demonstrate the presence of severe traumatic axonopathy in multiple ascending and descending long tracts after IA-TBI with features consistent with some chronic axonopathies and models of WD and the across-tract protective effect of Sarm1 deletion.
Keywords: Corticospinal tract; Electron microscopy; Microglia; Myelin; Neurodegeneration; Neuropathy; Oligodendrocyte; Traumatic axonal injury; Wallerian degeneration.
Copyright © 2022 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest None.
Figures




Similar articles
-
Traumatic Axonal Injury in the Optic Nerve: The Selective Role of SARM1 in the Evolution of Distal Axonopathy.J Neurotrauma. 2023 Aug;40(15-16):1743-1761. doi: 10.1089/neu.2022.0416. Epub 2023 Mar 14. J Neurotrauma. 2023. PMID: 36680758 Free PMC article.
-
Primary Traumatic Axonopathy in Mice Subjected to Impact Acceleration: A Reappraisal of Pathology and Mechanisms with High-Resolution Anatomical Methods.J Neurosci. 2018 Apr 18;38(16):4031-4047. doi: 10.1523/JNEUROSCI.2343-17.2018. Epub 2018 Mar 22. J Neurosci. 2018. PMID: 29567804 Free PMC article.
-
Sarm1 deletion reduces axon damage, demyelination, and white matter atrophy after experimental traumatic brain injury.Exp Neurol. 2019 Nov;321:113040. doi: 10.1016/j.expneurol.2019.113040. Epub 2019 Aug 21. Exp Neurol. 2019. PMID: 31445042
-
Myelin and oligodendrocyte lineage cells in white matter pathology and plasticity after traumatic brain injury.Neuropharmacology. 2016 Nov;110(Pt B):654-659. doi: 10.1016/j.neuropharm.2015.04.029. Epub 2015 May 9. Neuropharmacology. 2016. PMID: 25963414 Review.
-
White matter involvement after TBI: Clues to axon and myelin repair capacity.Exp Neurol. 2016 Jan;275 Pt 3:328-333. doi: 10.1016/j.expneurol.2015.02.011. Epub 2015 Feb 16. Exp Neurol. 2016. PMID: 25697845 Review.
Cited by
-
Application of P7C3 Compounds to Investigating and Treating Acute and Chronic Traumatic Brain Injury.Neurotherapeutics. 2023 Oct;20(6):1616-1628. doi: 10.1007/s13311-023-01427-8. Epub 2023 Aug 31. Neurotherapeutics. 2023. PMID: 37651054 Free PMC article. Review.
-
The transcriptional response of cortical neurons to concussion reveals divergent fates after injury.bioRxiv [Preprint]. 2024 Sep 17:2024.02.26.581939. doi: 10.1101/2024.02.26.581939. bioRxiv. 2024. Update in: Nat Commun. 2025 Jan 27;16(1):1097. doi: 10.1038/s41467-025-56292-0. PMID: 38463961 Free PMC article. Updated. Preprint.
-
NAD+, Axonal Maintenance, and Neurological Disease.Antioxid Redox Signal. 2023 Dec;39(16-18):1167-1184. doi: 10.1089/ars.2023.0350. Epub 2023 Sep 7. Antioxid Redox Signal. 2023. PMID: 37503611 Free PMC article. Review.
-
Direct microglia replacement reveals pathologic and therapeutic contributions of brain macrophages to a monogenic neurological disease.Immunity. 2025 May 13;58(5):1254-1268.e9. doi: 10.1016/j.immuni.2025.03.019. Epub 2025 Apr 30. Immunity. 2025. PMID: 40311614
-
Neuroinflammation: An Oligodendrocentric View.Glia. 2025 Jun;73(6):1113-1129. doi: 10.1002/glia.70007. Epub 2025 Mar 10. Glia. 2025. PMID: 40059542 Review.
References
-
- Adams JH, Doyle D, Graham DI, Lawrence AE, McLellan DR, 1984. Diffuse axonal injury in head injuries caused by a fall. Lancet 2 (8417–8418), 1420–1422. - PubMed
-
- Armstrong RC, Mierzwa AJ, Marion CM, Sullivan GM, 2016. White matter involvement after TBI: clues to axon and myelin repair capacity. Exp. Neurol. 275 (Pt 3), 328–333. - PubMed
-
- Bando Y, Nomura T, Bochimoto H, Murakami K, Tanaka T, Watanabe T, Yoshida S, 2015. Abnormal morphology of myelin and axon pathology in murine models of multiple sclerosis. Neurochem. Int. 81, 16–27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

