The immunomodulatory functions of chromogranin A-derived peptide pancreastatin
- PMID: 36244579
- PMCID: PMC10760928
- DOI: 10.1016/j.peptides.2022.170893
The immunomodulatory functions of chromogranin A-derived peptide pancreastatin
Abstract
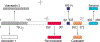
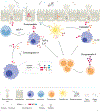
Chromogranin A (CgA) is a 439 amino acid protein secreted by neuroendocrine cells. Proteolytic processing of CgA results in the production of different bioactive peptides. These peptides have been associated with inflammatory bowel disease, diabetes, and cancer. One of the chromogranin A-derived peptides is ∼52 amino acid long Pancreastatin (PST: human (h)CgA250-301, murine (m)CgA263-314). PST is a glycogenolytic peptide that inhibits glucose-induced insulin secretion from pancreatic islet β-cells. In addition to this metabolic role, evidence is emerging that PST also has inflammatory properties. This review will discuss the immunomodulatory properties of PST and its possible mechanisms of action and regulation. Moreover, this review will discuss the potential translation to humans and how PST may be an interesting therapeutic target for treating inflammatory diseases.
Keywords: Chromogranin A (CgA); Inflammation; Macrophages; Pancreastatin (PST).
Copyright © 2022 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interest The authors declare no conflict of interest.
Figures

References
-
- Konecki DS, Benedum UM, Gerdes HH, Huttner WB, The primary structure of human chromogranin A and pancreastatin, J. Biol. Chem 262 (35) (1987) 17026–17030. - PubMed
-
- Mouland AJ, Bevan S, White JH, Hendy GN, Human chromogranin A gene. Molecular cloning, structural analysis, and neuroendocrine cell-specific expression, J. Biol. Chem 269 (9) (1994) 6918–6926. - PubMed
-
- Mosley CA, Taupenot L, Biswas N, et al., Biogenesis of the secretory granule: chromogranin A coiled-coil structure results in unusual physical properties and suggests a mechanism for granule core condensation, Biochemistry 46 (38) (2007) 10999–11012. - PubMed
-
- Murray SS, Deaven LL, Burton DW, O’Connor DI, Mellon PL, Deftos LJ, The gene for human chromogranin A (CgA) is located on chromosome 14, Biochem Biophys. Res. Commun 142 (1) (1987) 141–146. - PubMed
-
- Mahata SK, Kozak CA, Szpirer J, et al., Dispersion of chromogranin/ secretogranin secretory protein family loci in mammalian genomes, Genomics 33 (1) (1996) 135–139. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

