Whole-brain dynamics of human sensorimotor adaptation
- PMID: 36245212
- PMCID: PMC10110437
- DOI: 10.1093/cercor/bhac378
Whole-brain dynamics of human sensorimotor adaptation
Abstract
Humans vary greatly in their motor learning abilities, yet little is known about the neural processes that underlie this variability. We identified distinct profiles of human sensorimotor adaptation that emerged across 2 days of learning, linking these profiles to the dynamics of whole-brain functional networks early on the first day when cognitive strategies toward sensorimotor adaptation are believed to be most prominent. During early learning, greater recruitment of a network of higher-order brain regions, involving prefrontal and anterior temporal cortex, was associated with faster learning. At the same time, greater integration of this "cognitive network" with a sensorimotor network was associated with slower learning, consistent with the notion that cognitive strategies toward adaptation operate in parallel with implicit learning processes of the sensorimotor system. On the second day, greater recruitment of a network that included the hippocampus was associated with faster learning, consistent with the notion that declarative memory systems are involved with fast relearning of sensorimotor mappings. Together, these findings provide novel evidence for the role of higher-order brain systems in driving variability in adaptation.
Keywords: cognition; fMRI; learning; modularity; motor learning.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures
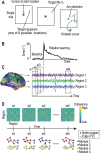

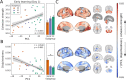
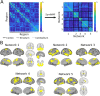
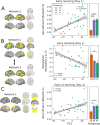

References
-
- Anguera JA, Russell CA, Noll DC, Seidler RD. Neural correlates associated with intermanual transfer of sensorimotor adaptation. Brain Res. 2007:1185:136–151. - PubMed
-
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Contributions of spatial working memory to visuomotor learning. J Cogn Neurosci. 2010:22(9):1917–1930. - PubMed
-
- Anguera JA, Reuter-Lorenz PA, Willingham DT, Seidler RD. Failure to engage spatial working memory contributes to age-related declines in visuomotor learning. J Cogn Neurosci. 2011:23(1):11–25. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

