A Flare Risk Index Informed by Select Immune Mediators in Systemic Lupus Erythematosus
- PMID: 36245261
- PMCID: PMC10106527
- DOI: 10.1002/art.42389
A Flare Risk Index Informed by Select Immune Mediators in Systemic Lupus Erythematosus
Abstract
Objective: Systemic lupus erythematosus (SLE) is marked by immune dysregulation linked to varied clinical disease activity. Using a unique longitudinal cohort of SLE patients, this study sought to identify optimal immune mediators informing an empirically refined flare risk index (FRI) reflecting altered immunity prior to clinical disease flare.
Methods: Thirty-seven SLE-associated plasma mediators were evaluated by microfluidic immunoassay in 46 samples obtained in SLE patients with an imminent clinical disease flare (preflare) and 53 samples obtained in SLE patients without a flare over a corresponding period (pre-nonflare). SLE patients were selected from a unique longitudinal cohort of 106 patients with classified SLE (meeting the American College of Rheumatology 1997 revised criteria for SLE or the Systemic Lupus International Collaborating Clinics 2012 revised criteria for SLE). Autoantibody specificities, hybrid SLE Disease Activity Index (hSLEDAI) scores, clinical features, and medication usage were also compared at preflare (mean ± SD 111 ± 47 days prior to flare) versus pre-nonflare (99 ± 21 days prior to nonflare) time points. Variable importance was determined by random forest analysis with logistic regression subsequently applied to determine the optimal number and type of analytes informing a refined FRI.
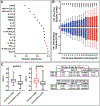
Results: Preflare versus pre-nonflare differences were not associated with demographics, autoantibody specificities, hSLEDAI scores, clinical features, nor medication usage. Forward selection and backward elimination of mediators ranked by variable importance resulted in 17 plasma mediator candidates differentiating preflare from pre-nonflare visits. A final combination of 11 mediators best informed a newly refined FRI, which achieved a maximum sensitivity of 97% and maximum specificity of 98% after applying decision curve analysis to define low, medium, and high FRI scores.
Conclusion: We verified altered immune mediators associated with imminent disease flare, and a subset of these mediators improved the FRI to identify SLE patients at risk of imminent flare. This molecularly informed, proactive management approach could be critical in prospective clinical trials and the clinical management of lupus.
© 2022 American College of Rheumatology.
Figures


References
-
- Steiman AJ, Gladman DD, Ibanez D, Urowitz MB. Prolonged serologically active clinically quiescent systemic lupus erythematosus: frequency and outcome. J Rheumatol. 2010;37(9):1822–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

