The disulfide catalyst QSOX1 maintains the colon mucosal barrier by regulating Golgi glycosyltransferases
- PMID: 36245281
- PMCID: PMC9841341
- DOI: 10.15252/embj.2022111869
The disulfide catalyst QSOX1 maintains the colon mucosal barrier by regulating Golgi glycosyltransferases
Abstract
Mucus is made of enormous mucin glycoproteins that polymerize by disulfide crosslinking in the Golgi apparatus. QSOX1 is a catalyst of disulfide bond formation localized to the Golgi. Both QSOX1 and mucins are highly expressed in goblet cells of mucosal tissues, leading to the hypothesis that QSOX1 catalyzes disulfide-mediated mucin polymerization. We found that knockout mice lacking QSOX1 had impaired mucus barrier function due to production of defective mucus. However, an investigation on the molecular level revealed normal disulfide-mediated polymerization of mucins and related glycoproteins. Instead, we detected a drastic decrease in sialic acid in the gut mucus glycome of the QSOX1 knockout mice, leading to the discovery that QSOX1 forms regulatory disulfides in Golgi glycosyltransferases. Sialylation defects in the colon are known to cause colitis in humans. Here we show that QSOX1 redox control of sialylation is essential for maintaining mucosal function.
Keywords: colon; glycosyltransferases; mucus; redox homeostasis; sulfhydryl oxidase.
© 2022 The Authors. Published under the terms of the CC BY 4.0 license.
Figures
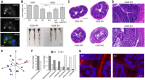
Immunofluorescence labeling of QSOX1 and a Golgi marker (GM130) in epithelial cells isolated from murine colon. QSOX1 is green, GM130 is red, and DAPI staining of nuclei is blue in the merged image. Scale bar is 10 μm.
Top, average colon length of WT and QSOX1 KO mice following indicated treatments. For untreated mice, colon lengths were calculated for 3 WT and 3 KO. For treated mice, lengths were calculated from 5 mice except for the DSS‐treated WT, for which 3 mice were analyzed. Error bars are standard deviation. Statistical analysis was performed using Tukey multiple comparison of means (**P < 0.01). Administration of QSOX1 inhibitory antibody MAb316.1 (αQSOX1, Grossman et al, ; Feldman et al, 2020) did not enhance the sensitivity of WT colons to DSS (P > 0.4 for MAb316.1‐treated WT vs. WT control antibody (IgG)‐treated; P > 0.5 for MAb316.1‐treated WT vs. WT without antibody treatment). Bottom, representative images of colons following DSS treatment. Scale bar is 1 cm.
Representative colon cross‐sections stained with H&E show severe damage to the epithelium in DSS‐treated KO mice. Scale bar is 500 μm.
Damage to epithelium and immune cell infiltration in representative DSS‐treated QSOX1 KO colons. Scale bar is 50 μm.
Principal coordinate plot of weighted Unifrac data from amplicon sequencing of feces from 5 WT and 5 KO mice using 16S universal eubacterial primers. The three vectors presented exhibit more than 70% of the variation among the groups. ANOSIM R statistic is 0.3, P‐value 0.011.
Mean percentage is displayed for genera that differ significantly between WT and KO fecal samples and that represent at least 1% of the sequences for either genotype. This experiment was done once, with 6 mice per group.
Fluorescence in situ hybridization of 16S rRNA (red). Blue indicates DAPI staining of nuclei. Scale bar is 10 μm. Representative images are shown from labeling of 5 WT and 5 KO mice. The experiment was performed twice on different sections from the same set of mice.
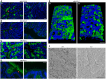
Cross‐sections of WT and KO colons immunolabeled (green) for Muc2 with an antibody recognizing the D3 assembly in the amino‐terminal segment (αMuc2‐D3; Javitt et al, 2020a). Blue is DAPI staining of nuclei. Orange arrowheads indicate secreted mucin. Scale bar is 20 μm and applies also to panels (B and C).
As in panel (A) except using an antibody recognizing the D1 assembly in the amino‐terminal segment (αMuc2‐D1). The antibody was raised against the human ortholog but is cross‐reactive with murine Muc2.
As in panel (A) except using an antibody recognizing the Muc2 carboxy terminus (αMuc2‐C).
A higher magnification image of a WT colon cross‐section shows a sharp line of Muc2 coating the epithelium (orange arrowhead), covered by diffuse mucin labeling. These features are absent from KO colons. Scale bar is 10 μm. All labeling described above was performed at least 5 times on 5 colons of each genotype. Shown are representative images.
Immunolabeling (αMuc2‐C; green) of the lumen of longitudinally cut colon sections. Blue is DAPI staining of nuclei. Scale bar is 50 μm. Incomplete coverage of the colon lumen by mucin strands even in WT may be due to limitations of the fixation procedure. This experiment was done 3 times, and a representative image is shown.
SEM micrographs reveal thick mucus coating a WT colon in contrast to the exposed epithelial cell surface of a KO colon. Scale bar is 5 μm. This imaging was done with one sample per genotype, and a representative image is presented.
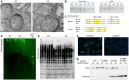
Representative TEM micrographs of about a hundred collected from three mice of each genotype show large and well‐packed goblet cell granules in WT and QSOX1 KO colons. Scale bar is 5 μm.
Alcian blue and PAS staining of reduced guanidine‐insoluble mucins from WT and QSOX1 KO mice, separated on a 6% polyacrylamide gel.
Amino acid sequences of murine Muc2 (Uniprot Q80Z19), Muc5b (Uniprot E9Q5I3), and Vwf (Uniprot Q8CIZ8) showing sequence conservation in the regions of the cysteines participating in intermolecular disulfide bonding to form polymers. Cysteines are highlighted in yellow, and asterisks indicate those that make intermolecular disulfides. Numbers indicate the amino acid positions of intermolecular disulfide‐bonding cysteines in the amino‐terminal region of murine Muc2, and in parentheses are corresponding amino acid positions in human MUC2, for reference to panel G.
Muc5b fluorescent immunoblot of WT and QSOX1 KO lung lavage samples separated on agarose gels.
Western blot analysis of blood Vwf from WT and QSOX1 KO mice separated on agarose gels.
QSOX1 immunofluorescence (green) in control (siControl) and QSOX1 knockdown (siQSOX1) MDA‐MB‐231 cells. Blue is DAPI staining of nuclei. Scale bar is 20 μm.
Western blot analysis of the MUC2 N‐terminal region and indicated cysteine mutants in supernatants of transfected cell cultures. No differences in disulfide‐mediated dimerization were observed for any MUC2 variant between siC (siControl) and siQ (siQSOX1).

Western blot analysis of colon epithelial cell lysates from WT and QSOX1 KO mice. Lysates were treated with PEG‐mal 2 kDa or NEM as indicated above each blot and probed using antibodies to St6gal1.
As for panel (A) but using antibodies to St3gal1.
As for panel (A) but using antibodies to B3galt5.
Structure of the human ST6GAL1 catalytic domain in complex with CMP (PDB ID: 4JS2; Kuhn et al, 2013) with cysteine side chains shown as spheres and numbered. Numbering according to the murine St6gal1 sequence is indicated in parentheses.
QSOX1 oxidizes St6gal1 in vitro. MAb492.1 is a monoclonal antibody that inhibits human QSOX1 (Grossman et al, 2013). St6gal1 with one pair of free cysteines is modified by two PEG‐mal additions (2PEG), whereas protein with two pairs of free cysteines acquires four PEG‐mal additions (4PEG). The change in migration per two PEG‐mal modifications appears greater than 4 kD as expected due to the differences in hydrodynamic properties and SDS binding of PEG vs. protein (Javitt et al, 2020b).
QSOX1 oxidation of St6gal1 mutants. The C350‐C361 disulfide (the redox‐active disulfide present in the C139A‐C403A mutant) is oxidized rapidly, while the C142‐C406 disulfide (present in the C350A‐C361A mutant) is oxidized slowly.

PAS and hematoxylin staining of colon cross sections. Scale bar is 50 μm.
Schematic showing the glycan species recognized by the indicated lectins. Stars represent fluorescent labels.
Colon cross sections labeled with SNA lectin. Scale bar is 100 μm.
Colon cross sections labeled with MAL II lectin. Scale bar is 100 μm.
Colon cross sections labeled with WGA lectin. Scale bar is 100 μm.
Comment in
-
A Golgi oxygen sensor controls intestinal mucin glycosylation.EMBO J. 2023 Jan 16;42(2):e113013. doi: 10.15252/embj.2022113013. Epub 2022 Nov 16. EMBO J. 2023. PMID: 36382686 Free PMC article.
References
-
- Antwi K, Hostetter G, Demeure MJ, Katchman BA, Decker GA, Ruiz Y, Sielaff TD, Koep LJ, Lake DF (2009) Analysis of the plasma peptidome from pancreas cancer patients connects a peptide in plasma to overexpression of the parent protein in tumors. J Protome Res 8: 4722–4731 - PubMed
-
- Appenzeller‐Herzog C, Ellgaard L (2008) The human PDI family: versatility packed into a single fold. Biochim Biophys Acta 1783: 535–548 - PubMed
-
- Baek JA, Song PH, Ko Y, Gu MJ (2018) High expression of QSOX1 is associated with tumor invasiveness and high grades groups in prostate cancer. Pathol Res Pract 214: 964–967 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

