Survival prognostic factors in unresectable/advanced primary thoracic sarcomas
- PMID: 36245612
- PMCID: PMC9562530
- DOI: 10.21037/jtd-22-472
Survival prognostic factors in unresectable/advanced primary thoracic sarcomas
Abstract
Background: Primary thoracic sarcomas (PTS) including primary pulmonary and chest wall sarcomas (CWS), are aggressive lung malignancies with limited information specially in an advanced/unresectable setting. Unfortunately, prognostic factors for these malignancies are not well identified.
Methods: Retrospective cohort analysis of patients diagnosed with unresectable/advanced soft tissue PTS from a third level reference institute. Univariate and multivariate analysis performed via Cox-regression model. Progression-free survival (PFS) and overall survival (OS) analysis via Kaplan-Meier method.
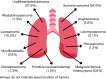
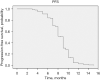
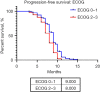
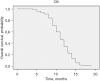
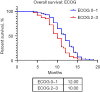
Results: A total of 157 patients were identified, 55.4% female, mean age 51.8 years (range, 18-90 years), 19.1% tobacco exposure and 10.8% asbestos exposure. The most common performance status was Eastern Cooperative Oncology Group (ECOG) 1 (38.9%), most common clinical presentation cough (58.4%) and thoracic pain (55.4%). Undifferentiated sarcoma (37.6%) followed by synovial sarcoma (34.4%) were the most common histologies. Most patients received five chemotherapeutic cycles (37.6%), 57.3% of patients obtained a partial response and 61.1% an overall response rate (ORR). Median PFS was 9 months [95% confidence interval (CI): 8.717-9.283 months]. The multivariable analysis identified ECOG ≥2, a poorer response to chemotherapy (less number of chemotherapy cycles) and an increase Response Evaluation Criteria in Solid Tumors (RECIST) to be associated with a shorter progression-free period. Median OS was 11 months (95% CI: 10.402-11.958 months) with an ECOG ≥2 and a poorer response to chemotherapy (less number of chemotherapy cycles) associated with a shorter survival.
Conclusions: Age, gender, comorbidities, tobacco and asbestos exposure, clinical presentation and histopathological diagnosis are not useful prognostic factors in unresectable/advanced PTS, however, an adequate initial ECOG, RECIST and a better response to chemotherapy should be used as prognostic factors in the management of these tumors.
Keywords: Primary thoracic sarcomas (PTS); overall survival (OS); prognostic factors; progression-free survival (PFS); thoracic oncology.
2022 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-472/coif). JRRC and JAAA have received research grants for other research protocols/projects, consulting fees working as speaker bureau and participating as advisory board for Astra Zeneca, Pfizer, Bayer, Roche, Roche Diagnostics, Novartis, Merch Sharp and Dohme (MSD), Bristol Myers Squibb (BMS), Takeda, Celltrion, Daiichi Sankyo, Novartis, GSK, Amgen, Eli Lilly. However, none of these are associated to the current study. The other authors have no conflicts of interest to declare.
Figures
References
-
- Fletcher CDM, Unni KK, Mertens F. World Health Organisation classification of tumours: pathology and genetics of tumours of soft tissue and bone. Lyon: IARC Press, 2002.
LinkOut - more resources
Full Text Sources
