Critical Role of Osteopontin in Maintaining Urinary Phosphate Solubility in CKD
- PMID: 36245654
- PMCID: PMC9528389
- DOI: 10.34067/KID.0007352021
Critical Role of Osteopontin in Maintaining Urinary Phosphate Solubility in CKD
Abstract
Background: Nephron loss dramatically increases tubular phosphate to concentrations that exceed supersaturation. Osteopontin (OPN) is a matricellular protein that enhances mineral solubility in solution; however, the role of OPN in maintaining urinary phosphate solubility in CKD remains undefined.
Methods: Here, we examined (1) the expression patterns and timing of kidney/urine OPN changes in CKD mice, (2) if tubular injury is necessary for kidney OPN expression in CKD, (3) how OPN deletion alters kidney mineral deposition in CKD mice, (4) how neutralization of the mineral-binding (ASARM) motif of OPN alters kidney mineral deposition in phosphaturic mice, and (5) the in vitro effect of phosphate-based nanocrystals on tubular epithelial cell OPN expression.
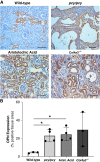
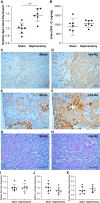
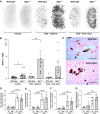
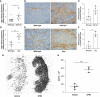
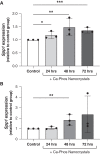
Results: Tubular OPN expression was dramatically increased in all studied CKD murine models. Kidney OPN gene expression and urinary OPN/Cr ratios increased before changes in traditional biochemical markers of kidney function. Moreover, a reduction of nephron numbers alone (by unilateral nephrectomy) was sufficient to induce OPN expression in residual nephrons and induction of CKD in OPN-null mice fed excess phosphate resulted in severe nephrocalcinosis. Neutralization of the ASARM motif of OPN in phosphaturic mice resulted in severe nephrocalcinosis that mimicked OPN-null CKD mice. Lastly, in vitro experiments revealed calcium-phosphate nanocrystals to induce OPN expression by tubular epithelial cells directly.
Conclusions: Kidney OPN expression increases in early CKD and serves a critical role in maintaining tubular mineral solubility when tubular phosphate concentrations are exceedingly high, as in late-stage CKD. Calcium-phosphate nanocrystals may be a proximal stimulus for tubular OPN production.
Keywords: basic science; chronic kidney disease; mineral metabolism; nephrocalcinosis; osteopontin; phosphate; solubility.
Copyright © 2022 by the American Society of Nephrology.
Conflict of interest statement
J. Boulanger reports being an employee of Sanofi-Genzyme. S. Liu reports being an employee of Sanofi-Genzyme and ownership interest in Sanofi. P.S. Rowe reports ownership interest in Apple (AAPL), CYBL, DWAC, and RTX, and patents or royalties from the University of Kansas Medical Center. J.R. Stubbs reports consultancy agreements with Novadiol; research funding from Genzyme; and being a scientific advisor for Spectradyne LLC. All remaining authors have nothing to disclose.
Figures


Comment in
-
Osteopontin Regulates Phosphate Solubility to Prevent Mineral Aggregates in CKD.Kidney360. 2022 Sep 29;3(9):1477-1479. doi: 10.34067/KID.0004292022. eCollection 2022 Sep 29. Kidney360. 2022. PMID: 36245661 Free PMC article. No abstract available.
References
-
- Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G; Kidney Disease: Improving Global Outcomes (KDIGO) : Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006. 10.1038/sj.ki.5000414 - DOI - PubMed
-
- Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011. 10.1038/ki.2011.47 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

