A new partially hydrolyzed whey-based follow-on formula with age-adapted protein content supports healthy growth during the first year of life
- PMID: 36245743
- PMCID: PMC9554543
- DOI: 10.3389/fped.2022.937882
A new partially hydrolyzed whey-based follow-on formula with age-adapted protein content supports healthy growth during the first year of life
Abstract
Background: Standard infant formulae often have higher protein content than breastmilk in order to compensate for potentially lower digestibility; excess protein intake may promote adverse effects later in life. A new partially hydrolyzed whey-based (pHF-W) follow-on formula (FoF) with age-adapted protein content was evaluated for growth and gastrointestinal (GI) tolerance in healthy infants.
Methods: Formula-fed (FF) infants (n = 108) received standard pHF-W formula (1.9 g protein/100 kcal) from enrollment (age ≤ 30 days) until age 120 days followed by new pHF-W FoF (1.6 g protein/100 kcal) until 360 days. Weight gain velocity (WGV) (mean daily WG from enrollment to age 180 days) was compared to WHO growth standards and a breastfed (BF) reference group (n = 86) (non-inferiority margin -3 g/day). GI tolerance was assessed using a validated questionnaire (scale range 13-65).
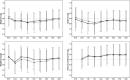
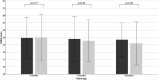
Results: WGV in FF infants (mean ± SD 24.0 ± 4.4 g/day) was non-inferior to BF (23.7 ± 3.9 g/day) and WHO standards (all p ≤ 0.013). Weight-for-age, length-for-age, weight-for-length, and head circumference-for-age z-scores of FF infants were not significantly different from BF at any timepoint. Symptoms of GI intolerance were low (≤23) at all timepoints and similar between groups.
Conclusion: A new pHF-W FoF with age-adapted protein content fed sequentially after standard pHF-W infant formula is safe, well-tolerated, and promotes a healthy growth pattern consistent with BF infants and WHO standards during the first year of life.
Clinical trial registration: [https://clinicaltrials.gov/], identifier [NCT03276663].
Keywords: France; follow-on formula; growth; infant formula; partially-hydrolyzed; protein; tolerance.
Copyright © 2022 Billeaud, Adamon, Piloquet, Hays, Dupuis, Metreau and Léké.
Conflict of interest statement
Author NH was employed by Société des Produits Nestlé S.A. Author LD was employed by Nestlé at the time of completion of this work. Author IM was employed by the Biofortis Research. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Centers for Disease Control and Prevention. National Center for Health Statistics. CDC Growth Charts; (2000). Available online at: http://www.cdc.gov/growthcharts/ (accessed August 14, 2022).
-
- Kliegman R, Stanton B, St Geme JW, Schor NF, Behrman RE, Nelson WE. The First Year. Nelson Textbook of Pediatrics. Philadelphia, PA: Elsevier; (2016).
-
- Dupont C. Protein requirements during the first year of life. Am J Clin Nutr. (2003) 77:1544S–9S. - PubMed
-
- Lonnerdal B. Infant formula and infant nutrition: bioactive proteins of human milk and implications for composition of infant formulas. Am J Clin Nutr. (2014) 99:712S–7S. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

