Determination of Benzalkonium Chloride in a Disinfectant by UV Spectrophotometry and Gas and High-Performance Liquid Chromatography: Validation, Comparison of Characteristics, and Economic Feasibility
- PMID: 36245786
- PMCID: PMC9553673
- DOI: 10.1155/2022/2932634
Determination of Benzalkonium Chloride in a Disinfectant by UV Spectrophotometry and Gas and High-Performance Liquid Chromatography: Validation, Comparison of Characteristics, and Economic Feasibility
Abstract
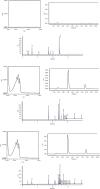
Simple, fast, and validated UV-spectrophotometric, HPLC, and GC methods for the analysis of benzalkonium chloride in a disinfectant were developed. UV-spectrophotometric determination was based on measuring the amount of light absorption of aqueous solutions of benzalkonium chloride at 268 nm. HPLC determination was achieved with a 150 mm × 4.6 mm, 5.0 μm C18 column. The mobile phase consisted of a 0.01% water solution of triethylamine (with pH 2.5) and acetonitrile in the ratio of 40 : 60 v/v. The column temperature was kept at 30°C, and the injection volume was 10 μL. The flow rate was 1.0 mL/min, and the diode array detector was set at 215 nm. GC determination was performed using a flame ionization detector on a glass capillary column ZB-WAX plus 30 m × 0.25 mm with an inner coating thickness of 0.25 μm. It creates a gradient increase in the temperature of the furnace to the maximum of 200°C. The temperature of the injector was 250°C, 1 μl was injected, and the separation of the sample was 1 : 100. Helium was used as a carrier gas, and the gas flow rate was constant, equaling 1.6 ml/min. The temperature of the detector was 250°C, and a mixture of hydrogen, helium, and air in a ratio of 30 : 8.5 : 350 was used in the split of the detector. All proposed methods were validated according to ICH guidelines with respect to the accuracy, precision (interday, intraday, and reproducibility), linearity, the limit of detection, the limit of quantitation, and robustness. All three methods were linear (R 2 = 0.997-0.999) over a concentration range of 400-600 μg/ml for UV and 80-120 μg/ml for HPLC and GC, accurate (recovery was 98.4-101.7% for all methods), precise (RSD <2%), and robust. The cost of the analysis of one sample of the disinfectant for the content of benzalkonium chloride by three methods was calculated. The comparison of the obtained results facilitates a more efficient allocation of laboratory resources, depending on the goal.
Copyright © 2022 Mariia Smolinska et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
[Simultaneous determination of 3 benzalkonium chloride homologs in cosmetics by high performance liquid chromatography].Se Pu. 2011 May;29(5):458-61. doi: 10.3724/sp.j.1123.2011.00458. Se Pu. 2011. PMID: 21847984 Chinese.
-
Development and validation of column high-performance liquid chromatographic and ultraviolet spectrophotometric methods for citalopram in tablets.J AOAC Int. 2008 Jan-Feb;91(1):52-8. J AOAC Int. 2008. PMID: 18376585
-
Development and validation of a HPLC method for the determination of cyclosporine a in new bioadhesive nanoparticles for oral administration.Indian J Pharm Sci. 2014 Mar;76(2):132-7. Indian J Pharm Sci. 2014. PMID: 24843186 Free PMC article.
-
Investigation of the Presence of Sildenafil in Herbal Dietary Supplements by Validated HPLC Method.Turk J Pharm Sci. 2020 Feb;17(1):56-62. doi: 10.4274/tjps.galenos.2018.91249. Epub 2020 Feb 19. Turk J Pharm Sci. 2020. PMID: 32454761 Free PMC article.
-
Stability study of hydromorphone and bupivacaine mixture by HPLC-UV.Eur J Hosp Pharm. 2020 Mar;27(2):95-99. doi: 10.1136/ejhpharm-2018-001553. Epub 2018 Oct 15. Eur J Hosp Pharm. 2020. PMID: 32133135 Free PMC article.
Cited by
-
Green Dentistry: State of the Art and Possible Development Proposals.Dent J (Basel). 2025 Jan 16;13(1):38. doi: 10.3390/dj13010038. Dent J (Basel). 2025. PMID: 39851612 Free PMC article. Review.
-
Development of a sustainable procedure for smartphone-based colorimetric determination of benzalkonium chloride in pharmaceutical preparations.Heliyon. 2024 Apr 17;10(9):e28965. doi: 10.1016/j.heliyon.2024.e28965. eCollection 2024 May 15. Heliyon. 2024. PMID: 38694067 Free PMC article.
References
-
- Domagk G. A new class of disinfectant. German Medical Weekly . 1935;61
-
- Kovalenko V. N. Compendium 2015. Drugs . 2015 Kyiv: MORION. [in Ukrainian]
-
- Martin R., Soberon N., Vaneechoutte M., Florez A. B., Vazquez F., Suarez J. Characterization of indigenous vaginal lactobacilli from healthy women as probiotic candidates. International Microbiology . 2008;11 - PubMed
-
- Kaewsrichan J., Peeyananjarassri K., Kongprasertkit J. Selection and identification of anaerobic lactobacilli producing inhibitory compounds against vaginal pathogens. FEMS Immunology and Medical Microbiology . 2006;48 - PubMed
-
- Klare I., Konstabel C., Werner G., et al. Antimicrobial susceptibilities of Lactobacillus, Pediococcus and Lactococcus human isolates and cultures intended for probiotic or nutritional use/ Journal of Antimicrobial Chemotherapy . 2007;59 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous