The immune landscape of hepatocellular carcinoma-where we are?
- PMID: 36245826
- PMCID: PMC9555061
- DOI: 10.3892/ol.2022.13530
The immune landscape of hepatocellular carcinoma-where we are?
Abstract
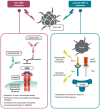
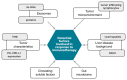
Hepatocellular carcinoma (HCC) is one of the most common types of cancer diagnosed worldwide. After a decade of stagnation, several novel compounds have recently been shown to be effective in the treatment of HCC. Since immunotherapy is associated with important clinical benefits in some, but not all patients, it is essential to identify reliable predictive biomarkers. As the complex interplay between hepatocytes and immune cells is highly dependent on the tumor microenvironment, the tumor microenviroment has been suggested to be an important factor associated with the response to therapy and is currently being extensively investigated. Within this network, several important factors should be highlighted. Most of the cells are hepatocytes, but fibroblasts, endothelial cells, and immune cells are also present. Tumor-infiltrating leukocytes include several populations of cells and each of them plays a role in forming the tumor environment. Some of these cells may have antitumor effects, whereas others may be associated with the progression of the disease. The most important subsets include tumor-associated macrophages, tumor-associated neutrophils, and lymphocytes. These groups are described in the present review. The immune response is controlled by immune checkpoint molecules. One of the most important molecules involved in this checkpoint process seems to be the programmed death-1 (PD-1) receptor, which typically is induced on activated T cells, natural killer (NK) cells, B cells, and antigen-presenting cells. On the other hand, programmed death ligand 1 (PD-L1) is expressed by tumor cells, hepatocytes and hepatic stellate cells, and Kupffer cells or liver sinusoidal cells. Complex interactions between ligands and receptors are dependent on the signals from the microenvironment leading to either cancer development or apoptosis. Evidence from several studies indicates that patients with higher expression levels of PD-L1 on tumor cells or immune cells are more likely to achieve beneficial results from treatment with checkpoint blockers. This review focuses on the basic information regarding the microenvironment and its components, particularly on immune system involvement.
Keywords: hepatocellular carcinoma; immunotherapy; tumour microenvironment; tumour-associated macrophages; tumour-infiltrating lymphocytes.
Copyright: © Gryziak et al.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Disruption of tumour-associated macrophage trafficking by the osteopontin-induced colony-stimulating factor-1 signalling sensitises hepatocellular carcinoma to anti-PD-L1 blockade.Gut. 2019 Sep;68(9):1653-1666. doi: 10.1136/gutjnl-2019-318419. Epub 2019 Mar 22. Gut. 2019. PMID: 30902885
-
Glycolytic activation of peritumoral monocytes fosters immune privilege via the PFKFB3-PD-L1 axis in human hepatocellular carcinoma.J Hepatol. 2019 Aug;71(2):333-343. doi: 10.1016/j.jhep.2019.04.007. Epub 2019 May 7. J Hepatol. 2019. PMID: 31071366
-
Platinum-based chemotherapy in combination with PD-1/PD-L1 inhibitors: preclinical and clinical studies and mechanism of action.Expert Opin Drug Deliv. 2021 Feb;18(2):187-203. doi: 10.1080/17425247.2021.1825376. Epub 2020 Oct 5. Expert Opin Drug Deliv. 2021. PMID: 32954856 Review.
-
Sensitizing tumors to anti-PD-1 therapy by promoting NK and CD8+ T cells via pharmacological activation of FOXO3.J Immunother Cancer. 2021 Dec;9(12):e002772. doi: 10.1136/jitc-2021-002772. J Immunother Cancer. 2021. PMID: 34887262 Free PMC article.
Cited by
-
The current status and future of PD-L1 in liver cancer.Front Immunol. 2023 Dec 12;14:1323581. doi: 10.3389/fimmu.2023.1323581. eCollection 2023. Front Immunol. 2023. PMID: 38155974 Free PMC article. Review.
-
Deciphering hepatocellular carcinoma pathogenesis and therapeutics: a study on anoikis, ceRNA regulatory network and traditional Chinese medicine.Front Pharmacol. 2024 Jan 12;14:1325992. doi: 10.3389/fphar.2023.1325992. eCollection 2023. Front Pharmacol. 2024. PMID: 38283837 Free PMC article.
-
From "Traditional" to "Trained" Immunity: Exploring the Novel Frontiers of Immunopathogenesis in the Progression of Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD).Biomedicines. 2025 Aug 18;13(8):2004. doi: 10.3390/biomedicines13082004. Biomedicines. 2025. PMID: 40868256 Free PMC article. Review.
-
Single-cell RNA sequencing reveals intratumoral heterogeneity and multicellular community in primary hepatocellular carcinoma underlying microvascular invasion.Heliyon. 2024 Aug 31;10(18):e37233. doi: 10.1016/j.heliyon.2024.e37233. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309949 Free PMC article.
-
Clinical significance of FBXO43 in hepatocellular carcinoma and its impact on tumor cell proliferation, migration and invasion.PeerJ. 2023 May 22;11:e15373. doi: 10.7717/peerj.15373. eCollection 2023. PeerJ. 2023. PMID: 37250703 Free PMC article.
References
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. doi: 10.1016/S1470-2045(08)70285-7. - DOI - PubMed
-
- Cheng A, Qin S, Ikeda M, Galle PR, Ducreux MP, Zhu AX, Kim T, Kudo M, Breder V, Merle P, et al. Imbrave150: Efficacy and safety results from a ph III study evaluating atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (Sor) as first treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC)'. Ann Oncol. 2019;30((Suppl 9)):ix183–ix202. doi: 10.1093/annonc/mdz446.002. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
