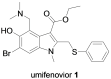
Synthesis and Biological Evaluation of Umifenovir Analogues as Anti-SARS-CoV-2 Agents
- PMID: 36245851
- PMCID: PMC9538379
- DOI: 10.1002/slct.202202097
Synthesis and Biological Evaluation of Umifenovir Analogues as Anti-SARS-CoV-2 Agents
Abstract
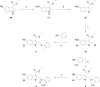
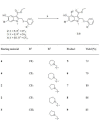
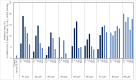
The unprecedented novel coronavirus disease 2019 (COVID-19) pandemic is a threat to global health and the economy. Since the outbreak of COVID-19, great effort has been made to reposition existing drugs to shorten development timelines, in addition to vaccine development and drug discovery campaigns. Umifenovir is a broad-spectrum antiviral agent used to treat influenza in China and Russia and is currently undergoing clinical trials for the treatment of COVID-19. In this article, the synthesis of umifenovir analogues and their biological evaluation are reported. The inhibitory activities of analogues against the binding of the spike glycoprotein (S-protein) of the novel severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) to the ACE2 receptor, which is a possible mode of action for umifenovir to inhibit viral infection, were investigated.
Keywords: ACE2 receptor; COVID-19; Indole; SARS-CoV-2; Umifenovir.
© 2022 Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- World Health Organization, “Weekly Operational Update on COVID-19”, 12–12-2021, https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio....
-
- None
-
- Zhou P., Yang X. L., Wang X. G., Hu B., Zhang L., Zhang W., Si H. R., Zhu Y., Li B., Huang C. L., Chen H. D., Chen J., Luo Y., Guo H., Jiang R. D., Liu M. Q., Chen Y., Shen X. R., Wang X., Zheng X. S., Zhao K., Chen Q. J., Deng F., Liu L. L., Yan B., Zhan F. X., Wang Y. Y., Xiao G. F., Shi Z. L., Nature 2020, 579, 270–273; - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
