[NEUROLOGICAL MANIFESTATIONS ASSOCIATED WITH COVID-19 VACCINE]
- PMID: 36245941
- PMCID: PMC9554338
- DOI: 10.1016/j.nrl.2022.09.005
[NEUROLOGICAL MANIFESTATIONS ASSOCIATED WITH COVID-19 VACCINE]
Abstract
Introduction: Coronavirus disease 2019 (COVID-19) has spread rapidly, giving rise to a pandemic, causing significant morbidity and mortality. In this context, many vaccines have emerged to try to deal with this disease.
Objective: To review the reported cases of neurological manifestations after the application of COVID-19 vaccines, describing clinical, analytical and neuroimaging findings and health outcomes.
Methods: We carried out a review through bibliographic searches in PubMed.
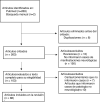
Results: We found 86 articles, including 13,809 patients with a wide spectrum of neurological manifestations temporally associated with COVID-19 vaccination. Most occurred in women (63.89%), with a median age of 50 years. The most frequently reported adverse events were Bell's palsy 4936/13809 (35.7%), headache (4067/13809), cerebrovascular events 2412/13809 (17.47%), Guillain-Barré syndrome 868/13809 (6.28%), central nervous system demyelination 258/13809 (1.86%) and functional neurological disorder 398/13809 (2.88%). Most of the published cases occurred in temporal association with the Pfizer vaccine (BNT162b2), followed by the AstraZeneca vaccine (ChAdOX1 nCoV-19).
Conclusions: It is not possible to establish a causal relationship between these adverse events and COVID-19 vaccines with the currently existing data, nor to calculate the frequency of appearance of these disorders. However, it is necessary for health professionals to be familiar with these events, facilitating their early diagnosis and treatment. Large controlled epidemiological studies are necessary to establish a possible causal relationship between vaccination against COVID-19 and neurological adverse events.
Introducción: La enfermedad por coronavirus 2019 (COVID-19) se ha propagado de forma rápida, dando lugar a una situación de pandemia, con importante morbimortalidad. En este contexto han surgido un amplio número de vacunas para tratar de hacer frente a la enfermedad.
Objetivo: Revisar los casos reportados de manifestaciones neurológicas tras la aplicación de las vacunas contra COVID-19, describiendo los hallazgos clínicos, analíticos, de neuroimagen y los resultados de salud.
Métodos: Revisión bibliográfica estructurada en la base de datos PubMed.
Resultados: Encontramos 86 artículos, que incluyeron 13.809 pacientes con un amplio espectro de manifestaciones neurológicas asociadas temporalmente con la vacunación contra COVID-19. La mayoría ocurrieron en mujeres (63,89%), con una mediana de edad de 50 años. Los eventos adversos publicados con más frecuencia fueron parálisis facial de Bell 4.936/13.809 (35,7%), cefalea (4.067/13.809), eventos vasculares cerebrales 2.412/13.809 (17,47%), síndrome de Guillain-Barré 868/13.809 (6,28%), desmielinización del sistema nervioso central 258/13.809 (1,86%) y trastorno neurológico funcional 398/13.809 (2,88%). La mayoría de casos publicados se produjeron en asociación temporal con la vacuna Pfizer (BNT162b2), seguida de la vacuna de AstraZeneca (ChAdOX1 nCoV-19).
Conclusiones: Con los datos existentes actualmente no es posible establecer una relación de causalidad entre estos eventos adversos y las vacunas contra la COVID-19, ni calcular la frecuencia de aparición de estos trastornos. Sin embargo, es necesario que los profesionales de la salud estén familiarizados con estos eventos, facilitando su diagnóstico y tratamiento tempranos. Son necesarios grandes estudios epidemiológicos controlados para establecer una posible relación causal entre la vacunación contra la COVID-19 y los eventos adversos neurológicos.
Keywords: COVID-19 vaccines; SARS-COV-2 vaccines; adverse event; neurological manifestations; neurological pathology; safety.
© 2022 Published by Elsevier España, S.L.U. on behalf of Sociedad Española de Neurología.
Figures
References
Bibliografía
References
-
- Graf T., Thiele T., Klingebiel R., Greinacher A., Rüdiger W. Immediate high-dose intravenous immunoglobulins followed by direct thrombin-inhibitor treatment is crucial for survival in Sars-Covid-19-adenoviral vector vaccine-induced immune thrombotic thrombocytopenia VITT with cerebral sinus venous and portal vein t. J Neurol. 2021;268:4483–4485. doi: 10.1007/s00415-021-10599-2. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous