Phase 1 Clinical Trial of Elamipretide in Dry Age-Related Macular Degeneration and Noncentral Geographic Atrophy: ReCLAIM NCGA Study
- PMID: 36246181
- PMCID: PMC9560640
- DOI: 10.1016/j.xops.2021.100086
Phase 1 Clinical Trial of Elamipretide in Dry Age-Related Macular Degeneration and Noncentral Geographic Atrophy: ReCLAIM NCGA Study
Abstract
Purpose: Assess the safety, tolerability, and feasibility of subcutaneous administration of the mitochondrial-targeted drug elamipretide in patients with dry age-related macular degeneration (AMD) and noncentral geographic atrophy (NCGA) and to perform exploratory analyses of change in visual function.
Design: Phase 1, single-center, open-label, 24-week clinical trial with preplanned NCGA cohort.
Participants: Adults ≥ 55 years of age with dry AMD and NCGA.
Methods: Participants received subcutaneous elamipretide 40-mg daily; safety and tolerability assessed throughout. Ocular assessments included normal-luminance best-corrected visual acuity (BCVA), low-luminance BCVA (LLBCVA), normal-luminance binocular reading acuity (NLBRA), low-luminance binocular reading acuity (LLBRA), spectral-domain OCT, fundus autofluorescence (FAF), and patient self-reported function by low-luminance questionnaire (LLQ).
Main outcome measures: Primary end point was safety and tolerability. Prespecified exploratory end-points included changes in BCVA, LLBCVA, NLBRA, LLBRA, geographic atrophy (GA) area, and LLQ.
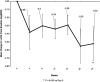
Results: Subcutaneous elamipretide was highly feasible. All participants (n = 19) experienced 1 or more nonocular adverse events (AEs), but all AEs were either mild (73.7%) or moderate (26.3%); no serious AEs were noted. Two participants exited the study because of AEs (conversion to neovascular AMD, n = 1; intolerable injection site reaction, n = 1), 1 participant discontinued because of self-perceived lack of efficacy, and 1 participant chose not to continue with study visits. Among participants completing the study (n = 15), mean ± standard deviation (SD) change in BCVA from baseline to week 24 was +4.6 (5.1) letters (P = 0.0032), while mean change (SD) in LLBCVA was +5.4 ± 7.9 letters (P = 0.0245). Although minimal change in NLBRA occurred, mean ± SD change in LLBCVA was -0.52 ± 0.75 logarithm of the minimum angle of resolution units (P = 0.005). Mean ± SD change in GA area (square root transformation) from baseline to week 24 was 0.14 ± 0.08 mm by FAF and 0.13 ± 0.14 mm by OCT. Improvement was observed in LLQ for dim light reading and general dim light vision.
Conclusions: Elamipretide seems to be well tolerated without serious AEs in patients with dry AMD and NCGA. Exploratory analyses demonstrated possible positive effect on visual function, particularly under low luminance. A Phase 2b trial is underway to evaluate elamipretide further in dry AMD and NCGA.
Keywords: AE, adverse event; AMD, age-related macular degeneration; BCVA, best-corrected visual acuity; Dry age-related macular degeneration; ETDRS, Early Treatment Diabetic Retinopathy Study; Elamipretide; FAF, fundus autofluorescence; GA, geographic atrophy; Geographic atrophy; LLBCVA, low-luminance best-corrected visual acuity; LLBRA, low-luminance binocular reading acuity; LLQ, low-luminance questionnaire; Mitochondrial dysfunction; NCGA, noncentral geographic atrophy; NLBRA, normal-luminance binocular reading acuity; Phase 1 clinical trial; RPE, retinal pigment epithelium; SD, standard deviation; logMAR, logarithm of the minimum angle of resolution.
© 2022 Published by Elsevier Inc. on behalf of the American Academy of Ophthalmology.
Figures


Similar articles
-
Phase 1 Clinical Trial of Elamipretide in Intermediate Age-Related Macular Degeneration and High-Risk Drusen: ReCLAIM High-Risk Drusen Study.Ophthalmol Sci. 2021 Dec 22;2(1):100095. doi: 10.1016/j.xops.2021.100095. eCollection 2022 Mar. Ophthalmol Sci. 2021. PMID: 36246187 Free PMC article.
-
ReCLAIM-2: A Randomized Phase II Clinical Trial Evaluating Elamipretide in Age-related Macular Degeneration, Geographic Atrophy Growth, Visual Function, and Ellipsoid Zone Preservation.Ophthalmol Sci. 2024 Oct 9;5(1):100628. doi: 10.1016/j.xops.2024.100628. eCollection 2025 Jan-Feb. Ophthalmol Sci. 2024. PMID: 39605874 Free PMC article.
-
A Phase I, Single Ascending Dose Study of GEM103 (Recombinant Human Complement Factor H) in Patients with Geographic Atrophy.Ophthalmol Sci. 2022 Apr 11;2(2):100154. doi: 10.1016/j.xops.2022.100154. eCollection 2022 Jun. Ophthalmol Sci. 2022. PMID: 36249705 Free PMC article.
-
Radiotherapy for neovascular age-related macular degeneration.Cochrane Database Syst Rev. 2020 Aug 26;8(8):CD004004. doi: 10.1002/14651858.CD004004.pub4. Cochrane Database Syst Rev. 2020. PMID: 32844399 Free PMC article.
-
The Progression of Geographic Atrophy Secondary to Age-Related Macular Degeneration.Ophthalmology. 2018 Mar;125(3):369-390. doi: 10.1016/j.ophtha.2017.08.038. Epub 2017 Oct 27. Ophthalmology. 2018. PMID: 29110945 Review.
Cited by
-
Role of Mitochondria in Retinal Pigment Epithelial Aging and Degeneration.Front Aging. 2022 Jul 15;3:926627. doi: 10.3389/fragi.2022.926627. eCollection 2022. Front Aging. 2022. PMID: 35912040 Free PMC article. Review.
-
Recent Advances in Our Understanding of Age-Related Macular Degeneration: Mitochondrial Dysfunction, Redox Signaling, and the Complement System.Aging Dis. 2024 Jan 24;16(3):1535-1575. doi: 10.14336/AD.2024.0124. Aging Dis. 2024. PMID: 38421830 Free PMC article. Review.
-
Molecular and Functional Characterization of BDNF-Overexpressing Human Retinal Pigment Epithelial Cells Established by Sleeping Beauty Transposon-Mediated Gene Transfer.Int J Mol Sci. 2022 Oct 26;23(21):12982. doi: 10.3390/ijms232112982. Int J Mol Sci. 2022. PMID: 36361771 Free PMC article.
-
The Mitochondria-Targeted Peptide Therapeutic Elamipretide Improves Cardiac and Skeletal Muscle Function During Aging Without Detectable Changes in Tissue Epigenetic or Transcriptomic Age.Aging Cell. 2025 Jun;24(6):e70026. doi: 10.1111/acel.70026. Epub 2025 Mar 13. Aging Cell. 2025. PMID: 40080911 Free PMC article.
-
Acute mitochondrial reactive oxygen species emissions drive mitochondrial dysfunction after traumatic muscle injury in male mice.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C235-C250. doi: 10.1152/ajpcell.00407.2025. Epub 2025 Jun 4. Am J Physiol Cell Physiol. 2025. PMID: 40465482 Free PMC article.
References
-
- Owsley C., McGwin G., Jackson G.R., et al. Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology. 2007;114:1728–1735. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

