YOSEMITE and RHINE: Phase 3 Randomized Clinical Trials of Faricimab for Diabetic Macular Edema: Study Design and Rationale
- PMID: 36246184
- PMCID: PMC9559760
- DOI: 10.1016/j.xops.2021.100111
YOSEMITE and RHINE: Phase 3 Randomized Clinical Trials of Faricimab for Diabetic Macular Edema: Study Design and Rationale
Abstract
Purpose: Faricimab is a novel anti-angiopoietin-2 and anti-vascular endothelial growth factor (VEGF) bispecific antibody with high affinities and specificities for both VEGF and angiopoietin-2. It is postulated that targeting angiogenic factors and inflammatory pathways in addition to the VEGF pathway will increase treatment durability and improve outcomes. The phase 3 YOSEMITE (ClinicalTrials.gov identifier, NCT03622580) and RHINE (ClinicalTrials.gov identifier, NCT03622593) trials are designed to assess efficacy, safety, and durability of faricimab compared with aflibercept in patients with diabetic macular edema (DME). The trials evaluate a personalized treatment interval (PTI) approach to address heterogeneity in treatment response among patients with DME.
Design: Two identically designed, global, double-masked, randomized, controlled phase 3 trials (YOSEMITE and RHINE).
Participants: Adults with center-involving DME secondary to type 1 or 2 diabetes mellitus.
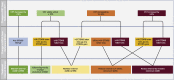
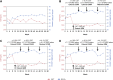
Methods: These studies were designed to evaluate 3 treatment groups: faricimab 6.0 mg dosed either at fixed dosing every 8 weeks after initial treatment with 6 intravitreal doses at 4-week intervals, or faricimab 6.0 mg dosed according to PTI after initial treatment with 4 every-4-week doses, compared with aflibercept 2.0 mg dosed every 8 weeks after 5 initial every-4-week doses. The primary end point of the studies was change from baseline in best-corrected visual acuity at 1 year, averaged over weeks 48, 52, and 56. Secondary end points included anatomic, durability, and patient-reported outcomes. Safety outcomes included incidence and severity of ocular and nonocular adverse events. The PTI is a protocol-defined flexible regimen based on the treat-and-extend concept, which allowed up to every-16-week adjustable dosing based on objective and standardized criteria. The PTI design aimed to maximize therapeutic results while minimizing treatment burden.
Main outcome measures: We describe the rationale for the study design and the novel PTI (up to every-16-week adjustable dosing) approach for treatment with faricimab.
Results: YOSEMITE and RHINE enrolled 940 and 951 patients, respectively. Results from each study will be reported separately.
Conclusions: YOSEMITE and RHINE were the first registrational trials in retinal disease to incorporate an objective PTI regimen, allowing for up to every-16-week adjustable dosing with a dual angiopoietin-2 and VEGF-A inhibitor, faricimab 6.0 mg, for treatment of DME.
Keywords: AE, adverse event; Adjustable dosing; Angiopoietin-2; Anti–vascular endothelial growth factor; BCVA, best-corrected visual acuity; Bispecific antibody; CFP, color fundus photography; CRC, central reading center; CST, central subfield thickness; DME, diabetic macular edema; DR, diabetic retinopathy; Diabetic macular edema; ETDRS, Early Treatment Diabetic Retinopathy Study; FFA, fundus fluorescein angiography; Faricimab; ITT, intention-to-treat; IxRS, interactive voice or web-based response system; PRN, pro re nata; PTI, personalized treatment interval; Personalized treatment interval; Phase 3 clinical trial design; SD, spectral-domain; T&E, treat-and-extend; VEGF, vascular endothelial growth factor.
© 2021 by the American Academy of Ophthalmology.
Figures


References
-
- Brown D.M., Schmidt-Erfurth U., Do D.V., et al. Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology. 2015;122:2044–2052. - PubMed
-
- Flaxel C.J., Adelman R.A., Bailey S.T., et al. Diabetic retinopathy Preferred Practice Pattern®. Ophthalmology. 2020;127:P66–P145. - PubMed
-
- Korobelnik J.-F., Do D.V., Schmidt-Erfurth U., et al. RESTORE study group Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121:2247–2254. - PubMed
-
- Mitchell P., Bandello F., Schmidt-Erfurth U., et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118:615–625. - PubMed
-
- Schmidt-Erfurth U., Garcia-Arumi J., Bandello F., et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA) Ophthalmologica. 2017;237:185–222. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

