Cryoelectron microscopy structures of a human neutralizing antibody bound to MERS-CoV spike glycoprotein
- PMID: 36246239
- PMCID: PMC9554411
- DOI: 10.3389/fmicb.2022.988298
Cryoelectron microscopy structures of a human neutralizing antibody bound to MERS-CoV spike glycoprotein
Abstract
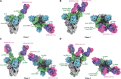
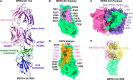
Neutralizing monoclonal antibodies (mAbs) against highly pathogenic coronaviruses represent promising candidates for clinical intervention. Here, we isolated a potent neutralizing monoclonal antibody, MERS-S41, from a yeast displayed scFv library using the S protein as a bait. To uncover the neutralization mechanism, we determined structures of MERS-S41 Fab in complex with the trimeric spike glycoprotein by cryoelectron microscopy (cryo-EM). We observed four distinct classes of the complex structure, which showed that the MERS-S41 Fab bound to the "up" receptor binding domain (RBD) with full saturation and also bound to an accessible partially lifted "down" RBD, providing a structural basis for understanding how mAbs bind to trimeric spike glycoproteins. Structure analysis of the epitope and cell surface staining assays demonstrated that virus entry is blocked predominantly by direct competition with the host receptor, dipeptidyl peptidase-4 (DPP4).
Keywords: MERS-CoV; cryo-EM structures; neutralization mechanism; neutralizing antibody; spike glycoprotein.
Copyright © 2022 Zhang, Jia, Zeng, Li, Wang, Zhou, Zhang and Wang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

