Efficacy and Safety of Inosine Pranobex in COVID-19 Patients: A Multicenter Phase 3 Randomized Double-Blind, Placebo-Controlled Trial
- PMID: 36246300
- PMCID: PMC9539257
- DOI: 10.1002/adtp.202200159
Efficacy and Safety of Inosine Pranobex in COVID-19 Patients: A Multicenter Phase 3 Randomized Double-Blind, Placebo-Controlled Trial
Abstract
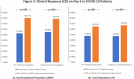
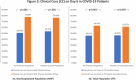
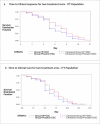
Inosine pranobex (IP), an immunomodulatory agent, is used in the treatment of various viral infections. The results of a phase 3 randomized controlled trial are reported, evaluating the efficacy and safety of IP in the treatment of mild to moderate COVID-19. It includes 416 symptomatic patients with confirmed SARS-CoV-2 infection. In addition to a defined standard of care, patients randomly (1:1) receive either IP 500 mg tablet (IP group) or a matching placebo (placebo group) at 50 mg kg-1 body weight/day rounded to the nearest 500 mg dose (maximum 4 g day-1) administered in 3-4 divided doses for 10 days. Compared to the placebo group, IP group shows significantly higher rates of clinical response (CR) and clinical cure (CC) on Day-6 for both non-hospitalized patients and the total population. IP group shows significantly earlier CR and CC with fewer adverse events and no mortality. Based on these findings and the fact that IP increases natural killer cell-mediated cytotoxicity of virus-infected cells as an early immune response to viral infection and enhances NKG2D ligand expression, it is concluded that IP should be started early to maximize the benefit in mild to moderate COVID-19 patients. (Trial registration number: CTRI/2021/02/030892).
Keywords: COVID‐19; antiviral; efficacy; inosine acedoben dimepranol; inosine pranobex; safety.
© 2022 Wiley‐VCH GmbH.
Conflict of interest statement
All the authors, except for A.S. and Y.B., acted as investigators in the clinical trial and have received their investigator's fees for this study. They have received no other funding related to the study. A.S. is an employee of Themis Medicare Limited, India, the sponsor of the study. Y.B. was an employee of Themis Medicare Limited, India when the study was conducted and when the first draft of the manuscript was prepared. He has checked and approved the final version of the manuscript being submitted. M.S.K. has received an honorarium from Themis Medicare Limited, India as a Speaker. All the authors report that they have no other disclosures to make.
Figures
References
-
- Central Drugs Standard Control Organization, List of new drugs approved in the year 2022 till date, https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadApprova... (accessed: April 2022).
-
- Rumel Ahmed S., Newman A. S., O'Daly J., Duffy S., Grafton G., Brady C. A., John Curnow S., Barnes N. M., Gordon J., Int. Immunopharmacol. 2017, 42,108. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
