Light-mediated control of gene expression in the anoxygenic phototrophic bacterium Rhodobacter capsulatus using photocaged inducers
- PMID: 36246361
- PMCID: PMC9561348
- DOI: 10.3389/fbioe.2022.902059
Light-mediated control of gene expression in the anoxygenic phototrophic bacterium Rhodobacter capsulatus using photocaged inducers
Abstract
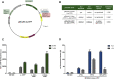
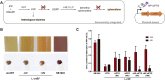
Photocaged inducer molecules, especially photocaged isopropyl-β-d-1-thiogalactopyranoside (cIPTG), are well-established optochemical tools for light-regulated gene expression and have been intensively applied in Escherichia coli and other bacteria including Corynebacterium glutamicum, Pseudomonas putida or Bacillus subtilis. In this study, we aimed to implement a light-mediated on-switch for target gene expression in the facultative anoxygenic phototroph Rhodobacter capsulatus by using different cIPTG variants under both phototrophic and non-phototrophic cultivation conditions. We could demonstrate that especially 6-nitropiperonyl-(NP)-cIPTG can be applied for light-mediated induction of target gene expression in this facultative phototrophic bacterium. Furthermore, we successfully applied the optochemical approach to induce the intrinsic carotenoid biosynthesis to showcase engineering of a cellular function. Photocaged IPTG thus represents a light-responsive tool, which offers various promising properties suitable for future applications in biology and biotechnology including automated multi-factorial control of cellular functions as well as optimization of production processes.
Keywords: Rhodobacter capsulatus; caged compounds; light-controlled gene expression; optogenetics; purple non-sulfur photosynthetic bacteria.
Copyright © 2022 Hilgers, Hogenkamp, Klaus, Kruse, Loeschcke, Bier, Binder, Jaeger, Pietruszka and Drepper.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Bier C., Binder D., Drobietz D., Loeschcke A., Drepper T., Jaeger K.-E., et al. (2016). Photocaged carbohydrates: Versatile tools for controlling gene expression by light. Synthesis 49, 42–52. 10.1055/s-0035-1562617 - DOI
-
- Binder D., Frohwitter J., Mahr R., Bier C., Grünberger A., Loeschcke A., et al. (2016). Light-controlled cell factories: Employing photocaged isopropyl-β-d-thiogalactopyranoside for light-mediated optimization of lac promoter-based gene expression and (+)-Valencene biosynthesis in Corynebacterium glutamicum . Appl. Environ. Microbiol. 82, 6141–6149. 10.1128/AEM.01457-16 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

