Anterior thalamic nuclei neurons sustain memory
- PMID: 36246504
- PMCID: PMC9559952
- DOI: 10.1016/j.crneur.2021.100022
Anterior thalamic nuclei neurons sustain memory
Abstract
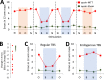
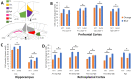
A hippocampal-diencephalic-cortical network supports memory function. The anterior thalamic nuclei (ATN) form a key anatomical hub within this system. Consistent with this, injury to the mammillary body-ATN axis is associated with examples of clinical amnesia. However, there is only limited and indirect support that the output of ATN neurons actively enhances memory. Here, in rats, we first showed that mammillothalamic tract (MTT) lesions caused a persistent impairment in spatial working memory. MTT lesions also reduced rhythmic electrical activity across the memory system. Next, we introduced 8.5 Hz optogenetic theta-burst stimulation of the ATN glutamatergic neurons. The exogenously-triggered, regular pattern of stimulation produced an acute and substantial improvement of spatial working memory in rats with MTT lesions and enhanced rhythmic electrical activity. Neither behaviour nor rhythmic activity was affected by endogenous stimulation derived from the dorsal hippocampus. Analysis of immediate early gene activity, after the rats foraged for food in an open field, showed that exogenously-triggered ATN stimulation also increased Zif268 expression across memory-related structures. These findings provide clear evidence that increased ATN neuronal activity supports memory. They suggest that ATN-focused gene therapy may be feasible to counter clinical amnesia associated with dysfunction in the mammillary body-ATN axis.
Keywords: Anterior thalamic nuclei; Episodic memory; Extended hippocampal system; Glutamatergic; Immediate early gene; Lesions; Mammillothalamc tract; Optogenetics; Recovery of function; Spatial memory; Theta; Working memory.
© 2021 Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



Similar articles
-
Anterior thalamic nuclei lesions have a greater impact than mammillothalamic tract lesions on the extended hippocampal system.Hippocampus. 2018 Feb;28(2):121-135. doi: 10.1002/hipo.22815. Epub 2017 Nov 29. Hippocampus. 2018. PMID: 29150979
-
Anterior thalamic nuclei: A critical substrate for non-spatial paired-associate memory in rats.Eur J Neurosci. 2022 Oct;56(7):5014-5032. doi: 10.1111/ejn.15802. Epub 2022 Aug 29. Eur J Neurosci. 2022. PMID: 35985792 Free PMC article.
-
Environmental enrichment increases prefrontal EEG power and synchrony with the hippocampus in rats with anterior thalamus lesions.Hippocampus. 2019 Feb;29(2):128-140. doi: 10.1002/hipo.23022. Epub 2018 Nov 22. Hippocampus. 2019. PMID: 30153381
-
Time to put the mammillothalamic pathway into context.Neurosci Biobehav Rev. 2021 Feb;121:60-74. doi: 10.1016/j.neubiorev.2020.11.031. Epub 2020 Dec 9. Neurosci Biobehav Rev. 2021. PMID: 33309908 Free PMC article. Review.
-
Anterior thalamic nuclei lesions and recovery of function: Relevance to cognitive thalamus.Neurosci Biobehav Rev. 2015 Jul;54:145-60. doi: 10.1016/j.neubiorev.2014.12.007. Epub 2015 Jan 29. Neurosci Biobehav Rev. 2015. PMID: 25637779 Review.
Cited by
-
Subcortical Alterations in Newly Diagnosed Epilepsy and Associated Changes in Brain Connectivity and Cognition.Hum Brain Mapp. 2024 Nov;45(16):e70069. doi: 10.1002/hbm.70069. Hum Brain Mapp. 2024. PMID: 39508641 Free PMC article. Review.
-
Molecular mechanisms underlying the neural correlates of working memory.BMC Biol. 2024 Oct 21;22(1):238. doi: 10.1186/s12915-024-02039-0. BMC Biol. 2024. PMID: 39428484 Free PMC article.
-
Anterior thalamic circuits crucial for working memory.Proc Natl Acad Sci U S A. 2022 May 17;119(20):e2118712119. doi: 10.1073/pnas.2118712119. Epub 2022 May 10. Proc Natl Acad Sci U S A. 2022. PMID: 35537049 Free PMC article.
-
Unraveling Brain Microcircuits, Dendritic Spines, and Synaptic Processing Using Multiple Complementary Approaches.Front Physiol. 2022 Feb 28;13:831568. doi: 10.3389/fphys.2022.831568. eCollection 2022. Front Physiol. 2022. PMID: 35295578 Free PMC article. No abstract available.
-
Time to retire the serial Papez circuit: Implications for space, memory, and attention.Neurosci Biobehav Rev. 2022 Sep;140:104813. doi: 10.1016/j.neubiorev.2022.104813. Epub 2022 Aug 5. Neurosci Biobehav Rev. 2022. PMID: 35940310 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

