The role of transcranial magnetic stimulation in understanding attention-related networks in single subjects
- PMID: 36246510
- PMCID: PMC9559099
- DOI: 10.1016/j.crneur.2021.100017
The role of transcranial magnetic stimulation in understanding attention-related networks in single subjects
Abstract
Attention is a cognitive mechanism that has been studied through several methodological viewpoints, including animal models, MRI in stroke patients, and fMRI in healthy subjects. Activation-based fMRI research has also pointed to specific networks that activate during attention tasks. Most recently, network neuroscience has been used to study the functional connectivity of large-scale networks for attention to reveal how strongly correlated networks are to each other when engaged in specific behaviors. While neuroimaging has revealed important information about the neural correlates of attention, it is crucial to better understand how these processes are organized and executed in the brain in single subjects to guide theories and treatments for attention. Noninvasive brain stimulation is an effective tool to causally manipulate neural activity to detect the causal roles of circuits in behavior. We describe how combining transcranial magnetic stimulation (TMS) with modern precision network analysis in single-subject neuroimaging could test the roles of regions, circuits, and networks in regulating attention as a pathway to improve treatment effect magnitudes and specificity.
Keywords: Attention; Network neuroscience; Network parcellation; Neuromodulation; Personalized neuromodulation; TMS.
© 2021 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



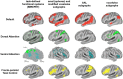

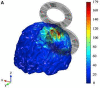




References
-
- Ahdab R., Ayache S.S., Brugieres P., Goujon C., Lefaucheur J.P. Comparison of “standard” and “navigated” procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiologie Clinique/Clin. Neurophysiol. 2010;40(1):27–36. - PubMed
-
- Anderson M.L. Neural reuse: a fundamental organizational principle of the brain. Behav. Brain Sci. 2010;33(4):245–266. - PubMed
-
- Aston-Jones G., Cohen J.D. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28:403–450. - PubMed
-
- Baluch F., Itti L. Mechanisms of top-down attention. Trends Neurosci. 2011;34(4):210–224. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

