Olfactory rule learning-induced enhancement in intrinsic neuronal excitability is maintained by shutdown of the cholinergic M-current
- PMID: 36246520
- PMCID: PMC9556983
- DOI: 10.3389/fncel.2022.934838
Olfactory rule learning-induced enhancement in intrinsic neuronal excitability is maintained by shutdown of the cholinergic M-current
Abstract
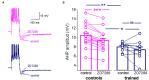
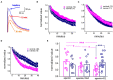
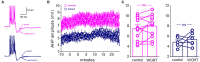
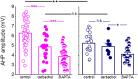
Training rats in a particularly difficult olfactory discrimination task initiates a period of accelerated learning, manifested as a dramatic increase in the rats' capacity to discriminate between pairs of odors once they have learned the discrimination task, implying that rule learning has taken place. At the cellular biophysical level, rule learning is maintained by reduction in the conductance of the slow current (sIAHP) simultaneously in most piriform cortex layer II pyramidal neurons. Such sIAHP reduction is expressed in attenuation of the post-burst afterhyperpolarization (AHP) potential and thus in enhanced repetitive action potential firing. Previous studies have shown that a causal relationship exists between long-lasting post-burst AHP reduction and rule learning. A specific channel through which the sIAHP flows has not been identified. The sIAHP in pyramidal cells is critically dependent on membrane phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P(2)]. PtdIns(4,5)P(2) regulates the calcium sensitivity of the sIAHP by acting downstream from the rise in intracellular calcium. These findings led to the interesting hypothesis that PtdIns(4,5)P(2) activates a variety of potassium channels. Thus, the sIAHP would not represent a unitary ionic current but the embodiment of a generalized potassium channel gating mechanism. We thus hypothesized that the learning-induced increase in intrinsic excitability is mediated by reduced conductance of one or more of the currents that contribute to the sIAHP. Here we first show, using current-clamp recordings, that the post-burst AHP in piriform cortex pyramidal neurons is also mediated by the Ih, and the contribution of this current to the post-burst AHP is also affected by learning. We also show, using whole-cell patch-clamp recordings, that the sIAHP in neurons from trained rats is not sensitive to blocking membrane phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P(2)], and to the blocking of the current mediated by the cholinergic muscarinic acetylcholine receptor (M-current). Further current-clamp recordings also show that blocking PtdIns(4,5)P(2) synthesis and application of a specific IKCa blocker have no effect on the post-burst AHP in neurons from trained as well as control rats. Taken together with results from our previous studies, these data suggest that rule learning-induced long-lasting enhancement in intrinsic neuronal excitability results from reduced conductance of the M-current and thus the slow potassium currents, which control repetitive spike firing.
Keywords: cholinergic modulation; intrinsic neuronal excitability; olfactory rule learning; piriform cortex; pyramidal neurons; slow afterhyperpolarization.
Copyright © 2022 Awasthi, Chandra and Barkai.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
A Cellular Mechanism Underlying Enhanced Capability for Complex Olfactory Discrimination Learning.eNeuro. 2019 Feb 12;6(1):ENEURO.0198-18.2019. doi: 10.1523/ENEURO.0198-18.2019. eCollection 2019 Jan-Feb. eNeuro. 2019. PMID: 30783614 Free PMC article.
-
A non-synaptic mechanism of complex learning: Modulation of intrinsic neuronal excitability.Neurobiol Learn Mem. 2018 Oct;154:30-36. doi: 10.1016/j.nlm.2017.11.015. Epub 2017 Nov 28. Neurobiol Learn Mem. 2018. PMID: 29196146 Review.
-
Long-lasting cholinergic modulation underlies rule learning in rats.J Neurosci. 2001 Feb 15;21(4):1385-92. doi: 10.1523/JNEUROSCI.21-04-01385.2001. J Neurosci. 2001. PMID: 11160410 Free PMC article.
-
Reversing Aging: Decline in Complex Olfactory Learning Can be Rectified by Restoring Intrinsic Plasticity of Hippocampal CA1 Pyramidal Neurons.Adv Biol (Weinh). 2024 Mar;8(3):e2300323. doi: 10.1002/adbi.202300323. Epub 2023 Dec 25. Adv Biol (Weinh). 2024. PMID: 38145360
-
Pharmacological and molecular enhancement of learning in aging and Alzheimer's disease.J Physiol Paris. 2006 Mar-May;99(2-3):180-92. doi: 10.1016/j.jphysparis.2005.12.079. Epub 2006 Feb 3. J Physiol Paris. 2006. PMID: 16458491 Review.
References
LinkOut - more resources
Full Text Sources

