Pharmacogenomics of drug transporters for antiretroviral long-acting pre-exposure prophylaxis for HIV
- PMID: 36246609
- PMCID: PMC9557974
- DOI: 10.3389/fgene.2022.940661
Pharmacogenomics of drug transporters for antiretroviral long-acting pre-exposure prophylaxis for HIV
Abstract
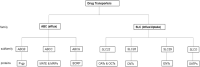
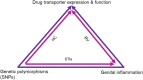
The use of antiretrovirals (ARVs) as oral, topical, or long-acting pre-exposure prophylaxis (PrEP) has emerged as a promising strategy for HIV prevention. Clinical trials testing Truvada® [tenofovir disoproxil fumarate (TDF)/tenofovir (TFV) and emtricitabine (FTC)] as oral or topical PrEP in African women showed mixed results in preventing HIV infections. Since oral and topical PrEP effectiveness is dependent on adequate drug delivery and availability to sites of HIV infection such as the blood and female genital tract (FGT); host biological factors such as drug transporters have been implicated as key regulators of PrEP. Drug transporter expression levels and function have been identified as critical determinants of PrEP efficacy by regulating PrEP pharmacokinetics across various cells and tissues of the blood, renal tissues, FGT mucosal tissues and other immune cells targeted by HIV. In addition, biological factors such as genetic polymorphisms and genital inflammation also influence drug transporter expression levels and functionality. In this review, drug transporters and biological factors modulating drug transporter disposition are used to explain discrepancies observed in PrEP clinical trials. This review also provides insight at a pharmacological level of how these factors further increase the susceptibility of the FGT to HIV infections, subsequently contributing to ineffective PrEP interventions in African women.
Keywords: African women; PrEP (pre-exposure prophylaxis); drug transporters; female genital tract (FGT); inflammation; single nucleotid polymorphism (SNP).
Copyright © 2022 Zondo, Sobia, Sivro, Ngcapu, Ramsuran and Archary.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Abdool Karim Q., Abdool Karim S. S. A., Frohlich J. A., Grobler A. C., Baxter C., Mansoor L. E., et al. (2010). Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. science 329, 1168–1174. 10.1126/science.1193748 - DOI - PMC - PubMed
-
- Abdool Karim Q., Kharsany A. B., Frohlich J. A., Werner L., Mlotshwa M., Madlala B. T., et al. (2012). HIV incidence in young girls in KwaZulu-Natal, South Africa-public health imperative for their inclusion in HIV biomedical intervention trials. AIDS Behav. 16, 1870–1876. 10.1007/s10461-012-0209-y - DOI - PMC - PubMed
-
- Alvarez E., Morello J., Soriano V., Labarga P., Rodriguez-Nóvoa S. (2011). Critical appraisal and update on tenofovir in management of human immunodeficiency virus infection. Virus Adapt. Treat. 3, 55. 10.2147/VAAT.S12708 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

