The role of GABA in islet function
- PMID: 36246925
- PMCID: PMC9558271
- DOI: 10.3389/fendo.2022.972115
The role of GABA in islet function
Erratum in
-
Erratum: The role of GABA in islet function.Front Endocrinol (Lausanne). 2023 Oct 2;14:1301830. doi: 10.3389/fendo.2023.1301830. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37850097 Free PMC article.
Abstract
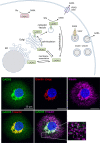
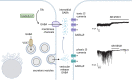
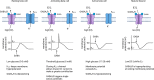
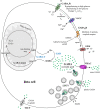
Gamma aminobutyric acid (GABA) is a non-proteinogenic amino acid and neurotransmitter that is produced in the islet at levels as high as in the brain. GABA is synthesized by the enzyme glutamic acid decarboxylase (GAD), of which the 65 kDa isoform (GAD65) is a major autoantigen in type 1 diabetes. Originally described to be released via synaptic-like microvesicles or from insulin secretory vesicles, beta cells are now understood to release substantial quantities of GABA directly from the cytosol via volume-regulated anion channels (VRAC). Once released, GABA influences the activity of multiple islet cell types through ionotropic GABAA receptors and metabotropic GABAB receptors. GABA also interfaces with cellular metabolism and ATP production via the GABA shunt pathway. Beta cells become depleted of GABA in type 1 diabetes (in remaining beta cells) and type 2 diabetes, suggesting that loss or reduction of islet GABA correlates with diabetes pathogenesis and may contribute to dysfunction of alpha, beta, and delta cells in diabetic individuals. While the function of GABA in the nervous system is well-understood, the description of the islet GABA system is clouded by differing reports describing multiple secretion pathways and effector functions. This review will discuss and attempt to unify the major experimental results from over 40 years of literature characterizing the role of GABA in the islet.
Keywords: beta cell; insulin; islet; pancreas; receptor; signaling; γ-Aminobutyric acid (GABA).
Copyright © 2022 Hagan, Ferreira, Santos and Phelps.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Okada Y, Taniguchi H, Shimada C, Kurosawa F. High concentration of γ-aminobutyric acid (GABA) in the langerhans’ islets of the pancreas. Procedings Japan Academy (1975) 51(10):760–2. doi: 10.2183/pjab1945.51.760 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

