Genetic Risk in Families with Age-Related Macular Degeneration
- PMID: 36246952
- PMCID: PMC9562327
- DOI: 10.1016/j.xops.2021.100087
Genetic Risk in Families with Age-Related Macular Degeneration
Abstract
Purpose: To determine the contribution of common and rare genetic risk variants in families with age-related macular degeneration (AMD).
Design: Case-control study.
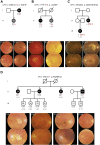
Participants: A family cohort (355 affected and 342 unaffected family members from 144 families with AMD) and an unrelated case-control cohort (1078 patients, 952 controls), recruited from the European Genetic Database.
Methods: Genetic data of both cohorts were filtered for carriership of rare genetic variants in the coding and splice-site regions of the complement factor H (CFH) and complement factor I (CFI) genes, and 52 AMD-associated variants were extracted for calculation of genetic risk scores (GRS). To compare GRSs between familial and nonfamilial rare CFH and CFI variant carriers and noncarriers and between AMD disease stages, we performed a 2-way analysis of variance, with Bonferroni correction for multiple testing. Within families with AMD carrying rare CFH and CFI variants, we analyzed segregation patterns by calculating the proportion of affected among carriers.
Main outcome measures: GRSs and segregation of rare CFH and CFI variants.
Results: We observed higher GRSs in familial versus nonfamilial individuals without rare CFH and CFI variants: mean GRS, 1.76 (standard error [SE], 0.08) versus 0.83 (SE, 0.03; P < 0.001). In 51 of 144 families (35.4%), rare CFH and CFI variants were identified. Within the AMD family cohort, carriers of rare CFH and CFI variants showed lower GRSs compared with noncarriers (mean GRS, 1.05 [SE, 0.23] vs. 1.76 [SE, 0.08]; P = 0.02). The proportion of affected family members with a high GRS was 57.3% (176/307). Of the affected family members with a low or intermediate GRS, 40.0% carried rare CFH or CFI variants. Among carriers of 11 rare CFH or CFI variants, the proportion affected by AMD was more than 75%.
Conclusions: Genetic risk in families with AMD often is attributed to high GRSs based on common variants. However, in part of the families with a low or intermediate GRS, rare CFH and CFI variants contributed to disease development. We recommend computing GRSs and sequencing the CFH and CFI genes in families with AMD, in particular in the light of ongoing gene-specific clinical trials.
Keywords: AMD, age-related macular degeneration; Age-related macular degeneration; CCP, complement control protein; CFH, complement factor H; CFI, complement factor I; CI, confidence interval; CIRCL, Cologne Image Reading Center and Laboratory; CNV, choroidal neovascularization; Complement factor H; Complement factor I; Complement system; GA, geographic atrophy; GRS, genetic risk score; GWAS, genome-wide association study; Genetic risk score; IQR, interquartile range; RC, Rotterdam Classification; SE, standard error.
© 2021 by the American Academy of Ophthalmology.
Figures




References
-
- Flaxman S.R., Bourne R.R.A., Resnikoff S., et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. - PubMed
-
- Meyers S.M., Greene T., Gutman F.A. A twin study of age-related macular degeneration. Am J Ophthalmol. 1995;120(6):757–766. - PubMed
-
- Seddon J.M., Cote J., Page W.F., et al. The US twin study of age-related macular degeneration: relative roles of genetic and environmental influences. Arch Ophthalmol. 2005;123(3):321–327. - PubMed
-
- Klaver C.C., Wolfs R.C., Assink J.J., et al. Genetic risk of age-related maculopathy. Population-based familial aggregation study. Arch Ophthalmol. 1998;116(12):1646–1651. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

