"Adipose-derived mesenchymal stem cell therapy for the management of female sexual dysfunction: Literature reviews and study design of a clinical trial"
- PMID: 36247008
- PMCID: PMC9554747
- DOI: 10.3389/fcell.2022.956274
"Adipose-derived mesenchymal stem cell therapy for the management of female sexual dysfunction: Literature reviews and study design of a clinical trial"
Abstract
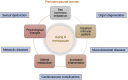
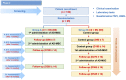
Hormone imbalance and female sexual dysfunction immensely affect perimenopausal female health and quality of life. Hormone therapy can improve female hormone deficiency, but long-term use increases the risk of cardiovascular diseases and cancer. Therefore, it is necessary to develop a novel effective treatment to achieve long-term improvement in female general and sexual health. This study reviewed factors affecting syndromes of female sexual dysfunction and its current therapy options. Next, the authors introduced research data on mesenchymal stromal cell/mesenchymal stem cell (MSC) therapy to treat female reproductive diseases, including Asherman's syndrome, premature ovarian failure/primary ovarian insufficiency, and vaginal atrophy. Among adult tissue-derived MSCs, adipose tissue-derived stem cells (ASCs) have emerged as the most potent therapeutic cell therapy due to their abundant presence in the stromal vascular fraction of fat, high proliferation capacity, superior immunomodulation, and strong secretion profile of regenerative factors. Potential mechanisms and side effects of ASCs for the treatment of female sexual dysfunction will be discussed. Our phase I clinical trial has demonstrated the safety of autologous ASC therapy for women and men with sexual hormone deficiency. We designed the first randomized controlled crossover phase II trial to investigate the safety and efficacy of autologous ASCs to treat female sexual dysfunction in perimenopausal women. Here, we introduce the rationale, trial design, and methodology of this clinical study. Because aging and metabolic diseases negatively impact the bioactivity of adult-derived MSCs, this study will use ASCs cultured in physiological oxygen tension (5%) to cope with these challenges. A total of 130 perimenopausal women with sexual dysfunction will receive two intravenous infusions of autologous ASCs in a crossover design. The aims of the proposed study are to evaluate 1) the safety of cell infusion based on the frequency and severity of adverse events/serious adverse events during infusion and follow-up and 2) improvements in female sexual function assessed by the Female Sexual Function Index (FSFI), the Utian Quality of Life Scale (UQOL), and the levels of follicle-stimulating hormone (FSH) and estradiol. In addition, cellular aging biomarkers, including plasminogen activator inhibitor-1 (PAI-1), p16 and p21 expression in T cells and the inflammatory cytokine profile, will also be characterized. Overall, this study will provide essential insights into the effects and potential mechanisms of ASC therapy for perimenopausal women with sexual dysfunction. It also suggests direction and design strategies for future research.
Keywords: ASCs (adipose stem cells); adipose-derived mesenchymal stem cells; cell therapy; female sexual dysfunction; menopause; regenerative medicine; sexual function impairment.
Copyright © 2022 Hoang, Nguyen, Nguyen, Hoang, Nguyen and Nguyen Thanh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Afifi N. M., Reyad O. N. (2013). Role of mesenchymal stem cell therapy in restoring ovarian function in a rat model of chemotherapy-induced ovarian failure. Egypt. J. Histology 36 (1), 114–126. 10.1097/01.EHX.0000423979.18253.10 - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

