Meis2 controls skeletal formation in the hyoid region
- PMID: 36247013
- PMCID: PMC9554219
- DOI: 10.3389/fcell.2022.951063
Meis2 controls skeletal formation in the hyoid region
Abstract
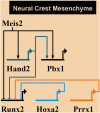
A vertebrate skull is composed of many skeletal elements which display enormous diversity of shapes. Cranial bone formation embodies a multitude of processes, i.e., epithelial-mesenchymal induction, mesenchymal condensation, and endochondral or intramembranous ossification. Molecular pathways determining complex architecture and growth of the cranial skeleton during embryogenesis are poorly understood. Here, we present a model of the hyoid apparatus development in Wnt1-Cre2-induced Meis2 conditional knock-out (cKO) mice. Meis2 cKO embryos develop an aberrant hyoid apparatus-a complete skeletal chain from the base of the neurocranium to lesser horns of the hyoid, resembling extreme human pathologies of the hyoid-larynx region. We examined key stages of hyoid skeletogenesis to obtain a complex image of the hyoid apparatus formation. Lack of Meis2 resulted in ectopic loci of mesenchymal condensations, ectopic cartilage and bone formation, disinhibition of skeletogenesis, and elevated proliferation of cartilage precursors. We presume that all these mechanisms contribute to formation of the aberrant skeletal chain in the hyoid region. Moreover, Meis2 cKO embryos exhibit severely reduced expression of PBX1 and HAND2 in the hyoid region. Altogether, MEIS2 in conjunction with PBX1 and HAND2 affects mesenchymal condensation, specification and proliferation of cartilage precursors to ensure development of the anatomically correct hyoid apparatus.
Keywords: Hand2; Meis2; PBX1; cartilage; hyoid bone; mesenchymal condensation.
Copyright © 2022 Fabik, Psutkova and Machon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Human fetal hyoid body origin revisited.J Anat. 2011 Aug;219(2):143-9. doi: 10.1111/j.1469-7580.2011.01387.x. Epub 2011 May 22. J Anat. 2011. PMID: 21599659 Free PMC article.
-
Conserved molecular program regulating cranial and appendicular skeletogenesis.Dev Dyn. 2004 Sep;231(1):4-13. doi: 10.1002/dvdy.20134. Dev Dyn. 2004. PMID: 15305282
-
Meis2 is essential for cranial and cardiac neural crest development.BMC Dev Biol. 2015 Nov 6;15:40. doi: 10.1186/s12861-015-0093-6. BMC Dev Biol. 2015. PMID: 26545946 Free PMC article.
-
Merging the old skeletal biology with the new. I. Intramembranous ossification, endochondral ossification, ectopic bone, secondary cartilage, and pathologic considerations.J Craniofac Genet Dev Biol. 2000 Apr-Jun;20(2):84-93. J Craniofac Genet Dev Biol. 2000. PMID: 11100738 Review.
-
Bone tissue and histological and molecular events during development of the long bones.Ann Anat. 2021 May;235:151704. doi: 10.1016/j.aanat.2021.151704. Epub 2021 Feb 16. Ann Anat. 2021. PMID: 33600952 Review.
Cited by
-
Transcriptional profiling of human cartilage endplate cells identifies novel genes and cell clusters underlying degenerated and non-degenerated phenotypes.Arthritis Res Ther. 2024 Jan 3;26(1):12. doi: 10.1186/s13075-023-03220-6. Arthritis Res Ther. 2024. PMID: 38173036 Free PMC article.
-
Mechanical stimulation in 2D: A potent accelerator of matrix mineralization in ATDC5 chondrogenic cells.J Orthop. 2025 May 31;70:173-182. doi: 10.1016/j.jor.2025.05.058. eCollection 2025 Dec. J Orthop. 2025. PMID: 40546983
References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

