Increased T- and B-cells associated with the phenotype of autoimmune limbic encephalitis with mainly memory dysfunction
- PMID: 36247087
- PMCID: PMC9563330
- DOI: 10.1016/j.jtauto.2022.100167
Increased T- and B-cells associated with the phenotype of autoimmune limbic encephalitis with mainly memory dysfunction
Abstract
Background: Our goal is to investigate the autoantibodies' presence and immune cells in the bioprobes of autoimmune encephalitis (AE) patients with distinct phenotypes as a promising target in AE.
Methods: We retrospectively analyzed immune cells via flow cytometry, serum and cerebrospinal fluid (CSF) autoantibodies, electroencephalography, magnetic resonance imaging in 94 AE patients with suspected temporal lobe epilepsy and classified neuropsychological phenotypes according to their occurrence.
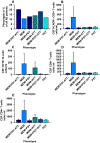
Results: We detected different phenotypes in 94 AE patients [10.6% with isolated memory dysfunction (MEM), 11.7% with mood-dysfunction, 12.7% with mood and memory dysfunction, 13.8% with memory and attention dysfunction, 18.1% with memory, mood and attention disturbances and 20.2% with no mood, memory or attention dysfunction]. We did discern a relevant association of phenotypes and CSF antibody-positivity on CSF CD4+ T-cells, CD8+T-cells and HLADR + CD8+T-cells in our patients with MEM presenting elevated CD8+T-cells and HLADR + CD8+T-cells. Furthermore, CSF CD19+B-cells differed significantly between phenotypes in patients with MEM.
Discussion: Taken together, the phenotypes in combination with CSF antibody-positivity are biomarkers for stratifying patients. Furthermore, our results confirm the role of CD4+ T-cells, CD8+T-cells and CD19+B-cells in AE patients with a memory dysfunction, providing insights into AE pathogenesis. Our preliminary results should be confirmed by larger-scale investigations.
Keywords: Autoantibodies; Autoimmune encephalitis; Immune cells; Neuropsychology; Phenotype; Verbal memory.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
References
-
- Graus F., Titulaer M.J., Balu R., Benseler S., Bien C.G., Celluci T., Cortese I., Dale R.C., Gelfand J.M., Geschwind M., Glaser C.A., Honnorat J., Höftberger R., Iizuka T., Irani S.R., Lancaster E., Leypoldt F., Prüss H., Rae-Grant A., Reindl M., Rosenfeld M.R., Rostásy K., Saiz A., Venkatesan A., Vincent A., Wandinger K.P., Waters P., Dalmau J. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. - DOI - PMC - PubMed
-
- Thompson J., Bi M., Murchison A.G., Makuch M., Bien C.G., Chu K., Farooque P., Gelfand J.M., Geschwind M.D., Hirsch L.J., Somerville E., Lang B., Vincent A., Leite M.I., Waters P., Irani S.R., Faciobrachial Dystonic Seizures Study Group Faciobrachial Dystonic Seizures Study Group. The importance of early immunotherapy in patients with faciobrachial dystonic seizures. Brain. 2018;141:348–356. doi: 10.1093/brain/awx323. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

