Discoidin domain receptor 2 expression increases phagocytotic capacity in sertoli cells of sertoli cell-only syndrome testes
- PMID: 36247266
- PMCID: PMC9556461
Discoidin domain receptor 2 expression increases phagocytotic capacity in sertoli cells of sertoli cell-only syndrome testes
Abstract
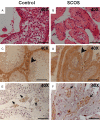
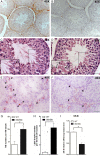
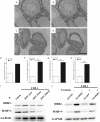
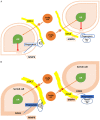
Discoidin domain receptor 2 (DDR2) belongs to the receptor tyrosine kinase (RTK) family, other RTKs have been reported to regulate phagocytic function of Sertoli cells (SCs), yet little is known about the function of DDR2 in Sertoli cells. In the present study, we aim to explore the function and mechanism of ectopic discoidin domain receptor 2 (DDR2) expression in Sertoli cells of Sertoli cell-only syndrome (SCOS) testes. We found that discoidin domain receptor 2 (DDR2) was absent in Sertoli cells of normal testis but was expressed in Sertoli cells of SCOS testes. This Sertoli cell DDR2 expression was induced by impaired androgen receptor (AR) signaling, but was inhibited by increased AR signaling from testosterone administration. The Sertoli cell DDR2 expression led to an increase in phagocytosis through up-regulation of Scavenger receptor class B member 1 (SR-BI) levels. However, loss of DDR2 by knock-out or knock-down weakened the phagocytotic capacity of Sertoli cells. Furthermore, the expression of DDR2 in Sertoli cells activated matrix metallopeptidase 9 (MMP-9) to consume abnormal collagen increase in seminiferous tubules which was responsible for the block of testosterone transportation and AR loss and to compensate for the impaired blood-testis-barrier (BTB). Our data suggest that the AR/DDR2 cascade may serve as a negative feedback mechanism to help compensate for the homeostasis of seminiferous epithelium in SCOS testis.
Keywords: Sertoli cell-only syndrome (SCOS); androgen receptor (AR); discoidin domain receptor 2 (DDR2); phagocytosis; sertoli cell.
AJTR Copyright © 2022.
Conflict of interest statement
None.
Figures



References
-
- Kierszenbaum AL. Mammalian spermatogenesis in vivo and in vitro: a partnership of spermatogenic and somatic cell lineages. Endocr Rev. 1994;15:116–134. - PubMed
-
- de Rooij DG, Grootegoed JA. Spermatogonial stem cells. Curr Opin Cell Biol. 1998;10:694–701. - PubMed
-
- Billig H, Furuta I, Rivier C, Tapanainen J, Parvinen M, Hsueh AJ. Apoptosis in testis germ cells: developmental changes in gonadotropin dependence and localization to selective tubule stages. Endocrinology. 1995;136:5–12. - PubMed
-
- Bartke A. Apoptosis of male germ cells, a generalized or a cell type-specific phenomenon? Endocrinology. 1995;136:3–4. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
