Sox13 is a novel flow-sensitive transcription factor that prevents inflammation by repressing chemokine expression in endothelial cells
- PMID: 36247423
- PMCID: PMC9561411
- DOI: 10.3389/fcvm.2022.979745
Sox13 is a novel flow-sensitive transcription factor that prevents inflammation by repressing chemokine expression in endothelial cells
Abstract
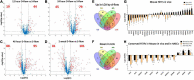
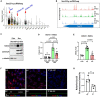
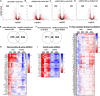
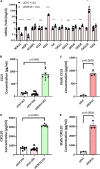
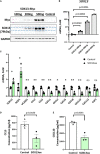
Atherosclerosis is a chronic inflammatory disease and occurs preferentially in arterial regions exposed to disturbed blood flow (d-flow) while the stable flow (s-flow) regions are spared. D-flow induces endothelial inflammation and atherosclerosis by regulating endothelial gene expression partly through the flow-sensitive transcription factors (FSTFs). Most FSTFs, including the well-known Kruppel-like factors KLF2 and KLF4, have been identified from in vitro studies using cultured endothelial cells (ECs). Since many flow-sensitive genes and pathways are lost or dysregulated in ECs during culture, we hypothesized that many important FSTFs in ECs in vivo have not been identified. We tested the hypothesis by analyzing our recent gene array and single-cell RNA sequencing (scRNAseq) and chromatin accessibility sequencing (scATACseq) datasets generated using the mouse partial carotid ligation model. From the analyses, we identified 30 FSTFs, including the expected KLF2/4 and novel FSTFs. They were further validated in mouse arteries in vivo and cultured human aortic ECs (HAECs). These results revealed 8 FSTFs, SOX4, SOX13, SIX2, ZBTB46, CEBPβ, NFIL3, KLF2, and KLF4, that are conserved in mice and humans in vivo and in vitro. We selected SOX13 for further studies because of its robust flow-sensitive regulation, preferential expression in ECs, and unknown flow-dependent function. We found that siRNA-mediated knockdown of SOX13 increased endothelial inflammatory responses even under the unidirectional laminar shear stress (ULS, mimicking s-flow) condition. To understand the underlying mechanisms, we conducted an RNAseq study in HAECs treated with SOX13 siRNA under shear conditions (ULS vs. oscillatory shear mimicking d-flow). We found 94 downregulated and 40 upregulated genes that changed in a shear- and SOX13-dependent manner. Several cytokines, including CXCL10 and CCL5, were the most strongly upregulated genes in HAECs treated with SOX13 siRNA. The robust induction of CXCL10 and CCL5 was further validated by qPCR and ELISA in HAECs. Moreover, the treatment of HAECs with Met-CCL5, a specific CCL5 receptor antagonist, prevented the endothelial inflammation responses induced by siSOX13. In addition, SOX13 overexpression prevented the endothelial inflammation responses. In summary, SOX13 is a novel conserved FSTF, which represses the expression of pro-inflammatory chemokines in ECs under s-flow. Reduction of endothelial SOX13 triggers chemokine expression and inflammatory responses, a major proatherogenic pathway.
Keywords: CCL5; CXCL10; Sox13; endothelium; inflammation; shear-sensitive TF.
Copyright © 2022 Demos, Johnson, Andueza, Park, Kim, Villa-Roel, Kang, Kumar and Jo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

