Stimulation of adventitious root formation by laser wounding in rose cuttings: A matter of energy and pattern
- PMID: 36247617
- PMCID: PMC9557736
- DOI: 10.3389/fpls.2022.1009085
Stimulation of adventitious root formation by laser wounding in rose cuttings: A matter of energy and pattern
Abstract
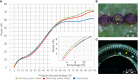
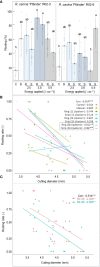
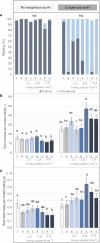
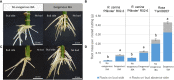
Adventitious root (AR) formation is the basis of vegetative propagation in rose, be it via stem cuttings or via stenting. During this process, wounding plays a pivotal role since cell reprogramming takes place at the tissue adjacent to the wound. We investigated the effects of wounding on AR formation on leafy single-node stem cuttings of the rose rootstock R. canina 'Pfänder' (codes R02-3 and R02-6) and the cut rose cultivar Rosa 'Tan09283' (Registration name 'Beluga'). Laser wounding treatments were based on the assisted removal of tissue layers located in the bark. The positioning of wounding was studied based on two marking directions: along the cutting base (strip pattern) and around the cutting base (ring pattern). Additionally, the effects of external supply of indole-butyric acid (IBA 1 mg L-1) on rooting were analyzed. Results showed that in order to remove specific tissue layers, the calculation of the laser energy density (J cm-2) in terms of cutting diameter was necessary. Interestingly, the application of energy densities from 2.5 J cm-2 up to approximately 8.5 J cm-2 were sufficient to expose the tissue layers of epidermis up to regions of phloem. Regarding AR formation for R. canina 'Pfänder', characterized by a low rooting response, an increase in the rooting percentage was registered when the laser treatment eliminated the tissue up to phloem proximities. Analysis of the nodal position showed that bud location was a preferential place for AR formation independently of wounding treatment. In case of Rosa 'Tan09283', laser treatments did not reduce its high rooting capacity, but an apparent reduction in rooting quality due to an investment in tissue healing was observed when wounding reached deeper layers such as parenchyma and sclerenchyma. Results also showed a strong AR formation directly from wounded regions in case of Rosa 'Tan09283' specifically when the wound was located below the axillary bud. In conclusion, wounding by assisted-elimination of layers by laser can induce positive effects on AR formation of single-node stem cuttings of the rose if energy applied is able to expose phloem proximities, a longitudinal orientation, and relative position to the axillary bud are considered.
Keywords: Rosa canina; adventitious root formation; histology; laser ablation; stem cutting; wounding.
Copyright © 2022 Morales-Orellana, Winkelmann, Bettin and Rath.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Laser-wound stimulated adventitious root formation of Rosa canina cuttings involves a complex response at plant hormonal and metabolic level.Front Plant Sci. 2024 Dec 16;15:1515990. doi: 10.3389/fpls.2024.1515990. eCollection 2024. Front Plant Sci. 2024. PMID: 39737379 Free PMC article.
-
Involvement of the auxin-cytokinin homeostasis in adventitious root formation of rose cuttings as affected by their nodal position in the stock plant.Planta. 2021 Sep 6;254(4):65. doi: 10.1007/s00425-021-03709-x. Planta. 2021. PMID: 34487248 Free PMC article.
-
Influence of light and shoot development stage on leaf photosynthesis and carbohydrate status during the adventitious root formation in cuttings of Corylus avellana L.Front Plant Sci. 2015 Nov 6;6:973. doi: 10.3389/fpls.2015.00973. eCollection 2015. Front Plant Sci. 2015. PMID: 26635821 Free PMC article.
-
Plant Hormone Homeostasis, Signaling, and Function during Adventitious Root Formation in Cuttings.Front Plant Sci. 2016 Mar 31;7:381. doi: 10.3389/fpls.2016.00381. eCollection 2016. Front Plant Sci. 2016. PMID: 27064322 Free PMC article. Review.
-
Hypocotyl adventitious root organogenesis differs from lateral root development.Front Plant Sci. 2014 Sep 29;5:495. doi: 10.3389/fpls.2014.00495. eCollection 2014. Front Plant Sci. 2014. PMID: 25324849 Free PMC article. Review.
Cited by
-
Anatomical limitations in adventitious root formation revealed by magnetic resonance imaging, infrared spectroscopy, and histology of rose genotypes with contrasting rooting phenotypes.J Exp Bot. 2024 Aug 28;75(16):4784-4801. doi: 10.1093/jxb/erae158. J Exp Bot. 2024. PMID: 38606898 Free PMC article.
-
Genome-wide association study (GWAS) analyses of early anatomical changes in rose adventitious root formation.Sci Rep. 2024 Oct 23;14(1):25072. doi: 10.1038/s41598-024-75502-1. Sci Rep. 2024. PMID: 39443540 Free PMC article.
-
Laser-wound stimulated adventitious root formation of Rosa canina cuttings involves a complex response at plant hormonal and metabolic level.Front Plant Sci. 2024 Dec 16;15:1515990. doi: 10.3389/fpls.2024.1515990. eCollection 2024. Front Plant Sci. 2024. PMID: 39737379 Free PMC article.
References
-
- Agulló-Antón M.Á., Ferrández-Ayela A., Fernández-García N., Nicolás C., Albacete A., Pérez-Alfocea F., et al. . (2014). Early steps of adventitious rooting: morphology, hormonal profiling and carbohydrate turnover in carnation stem cuttings. Physiol Plant 150, 446–462. doi: 10.1111/ppl.12114 - DOI - PubMed
-
- Ahkami A. H., Melzer M., Ghaffari M. R., Pollmann S., Ghorbani J. M., Shahinnia F. (2013). Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. Planta. 238, 499–517. doi: 10.1007/s00425-013-1907-z - DOI - PMC - PubMed
-
- Bourgault R., Matschi S., Vasquez M., Qiao P., Sonntag A., Charlebois C. (2020). Constructing functional cuticles: Analysis of relationships between cuticle lipid composition, ultrastructure and water barrier function in developing adult maize leaves. Ann. botany. 125, 79–91. doi: 10.1093/aob/mcz143 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

