Soil applied silicon and manganese combined with foliar application of 5-aminolevulinic acid mediate photosynthetic recovery in Cd-stressed Salvia miltiorrhiza by regulating Cd-transporter genes
- PMID: 36247621
- PMCID: PMC9558727
- DOI: 10.3389/fpls.2022.1011872
Soil applied silicon and manganese combined with foliar application of 5-aminolevulinic acid mediate photosynthetic recovery in Cd-stressed Salvia miltiorrhiza by regulating Cd-transporter genes
Abstract
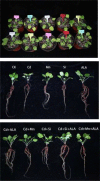
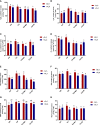
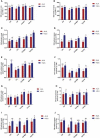
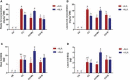
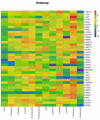
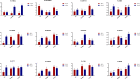
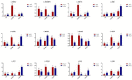
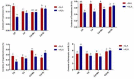
Salvia miltiorrhiza is an important medicinal plant that experiences significant growth and biomass losses when cultivated on cadmium (Cd) contaminated soils. High Cd accumulation in plant tissues also increases the risk of metal entry into the food chain. In this study, we proposed that Cd accumulation in S. miltiorrhiza can be restricted through plant growth regulators and nutrient management. Therefore, S. miltiorrhiza seedlings were transplanted into mixed nutrient soil for two weeks, then treated with 30 mg kg-1 CdCl2, 200 mg kg-1 Na2SiO3·9H2O, and 100 mg kg-1 MnSO4, and simultaneously sprayed with 10 mg L-1 ALA on the leaves one week later. This study showed that elevated Cd accumulation significantly reduced plant growth and biomass. This growth inhibition damaged photosynthetic machinery and impaired carbon assimilation. In contrast, 5-aminolevulinic acid (ALA) significantly promoted the biomass of S. miltiorrhiza, and the dry weight of plants treated with ALA combined with manganese (Mn)/silicon (Si) increased by 42% and 55% as compared with Cd+Mn and Cd+Si treatments. Exogenously applied ALA and Si/Mn significantly activated antioxidant enzymes and promoted the growth recovery of S. miltiorrhiza. Further, exogenous ALA also reduced the Cd concentration in S. miltiorrhiza, especially when combined with Si. Compared with the Cd+Si treatment, the Cd+Si+ALA treatment reduced the Cd concentration in roots and leaves by 59% and 60%, respectively. Gene expression analysis suggested that ALA and Si significantly up-regulated genes associated with Cd transport. Other genes related to heavy metal tolerance mechanisms are also regulated to cope with heavy metal stress. These results indicated that the combined action of ALA and Si/Mn could reduce Cd-toxicity by increasing chlorophyll content and changing oxidative stress and can also affect Cd accumulation by regulating gene expression.
Keywords: gene regulation; heavy metals; manganese; oxidative stress; plant growth regulator; silicon.
Copyright © 2022 Sun, Li, Najeeb, Hou, Buttar, Yang, Ali and Xu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer IK declared a past co-authorship with the author BA to the handling editor
Figures

Similar articles
-
Physiological and molecular mechanisms of ZnO quantum dots mitigating cadmium stress in Salvia miltiorrhiza.J Hazard Mater. 2024 May 15;470:134245. doi: 10.1016/j.jhazmat.2024.134245. Epub 2024 Apr 9. J Hazard Mater. 2024. PMID: 38603910
-
Elucidating silicon-mediated distinct morpho-physio-biochemical attributes and organic acid exudation patterns of cadmium stressed Ajwain (Trachyspermum ammi L.).Plant Physiol Biochem. 2020 Dec;157:23-37. doi: 10.1016/j.plaphy.2020.10.010. Epub 2020 Oct 10. Plant Physiol Biochem. 2020. PMID: 33069978
-
Silicon nanoparticles enhanced the growth and reduced the cadmium accumulation in grains of wheat (Triticum aestivum L.).Plant Physiol Biochem. 2019 Jul;140:1-8. doi: 10.1016/j.plaphy.2019.04.041. Epub 2019 May 3. Plant Physiol Biochem. 2019. PMID: 31078051
-
Sustainable management of cadmium-contaminated soils as affected by exogenous application of nutrients: A review.J Environ Manage. 2021 Oct 1;295:113081. doi: 10.1016/j.jenvman.2021.113081. Epub 2021 Jun 23. J Environ Manage. 2021. PMID: 34171783 Review.
-
Effects of silicon on heavy metal uptake at the soil-plant interphase: A review.Ecotoxicol Environ Saf. 2021 Oct 1;222:112510. doi: 10.1016/j.ecoenv.2021.112510. Epub 2021 Jul 14. Ecotoxicol Environ Saf. 2021. PMID: 34273846 Review.
Cited by
-
Abiotic stress-induced changes in Tetrastigma hemsleyanum: insights from secondary metabolite biosynthesis and enhancement of plant defense mechanisms.BMC Plant Biol. 2024 Dec 27;24(1):1260. doi: 10.1186/s12870-024-05975-9. BMC Plant Biol. 2024. PMID: 39725878 Free PMC article.
References
-
- Akram N. A., Ashraf M. (2013). Regulation in plant stress tolerance by a potential plant growth regulator, 5-aminolevulinic acid. J. Plant Growth Regulation 32, 663–679. doi: 10.1007/s00344-013-9325-9 - DOI
-
- Akram N. A., Ashraf M., Al-Qurainy F. (2012). Aminolevulinic acid-induced changes in some key physiological attributes and activities of antioxidant enzymes in sunflower (Helianthus annuus l.) plants under saline regime. Sci. Horticult. 142, 143–148. doi: 10.1016/j.scienta.2012.05.007 - DOI
-
- Ali B., Qian P., Jin R., Ali S., Khan M., Aziz R., et al. . (2014). Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biol. Plantarum. 58, 131–138. doi: 10.1007/s10535-013-0358-5 - DOI
LinkOut - more resources
Full Text Sources

