Probing the structure and function of locus coeruleus projections to CNS motor centers
- PMID: 36247730
- PMCID: PMC9556855
- DOI: 10.3389/fncir.2022.895481
Probing the structure and function of locus coeruleus projections to CNS motor centers
Abstract
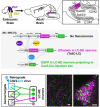
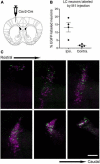
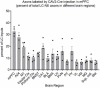
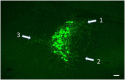
The brainstem nucleus locus coeruleus (LC) sends projections to the forebrain, brainstem, cerebellum and spinal cord and is a source of the neurotransmitter norepinephrine (NE) in these areas. For more than 50 years, LC was considered to be homogeneous in structure and function such that NE would be released uniformly and act simultaneously on the cells and circuits that receive LC projections. However, recent studies have provided evidence that LC is modular in design, with segregated output channels and the potential for differential release and action of NE in its projection fields. These new findings have prompted a radical shift in our thinking about LC operations and demand revision of theoretical constructs regarding impact of the LC-NE system on behavioral outcomes in health and disease. Within this context, a major gap in our knowledge is the relationship between the LC-NE system and CNS motor control centers. While we know much about the organization of the LC-NE system with respect to sensory and cognitive circuitries and the impact of LC output on sensory guided behaviors and executive function, much less is known about the role of the LC-NE pathway in motor network operations and movement control. As a starting point for closing this gap in understanding, we propose using an intersectional recombinase-based viral-genetic strategy TrAC (Tracing Axon Collaterals) as well as established ex vivo electrophysiological assays to characterize efferent connectivity and physiological attributes of mouse LC-motor network projection neurons. The novel hypothesis to be tested is that LC cells with projections to CNS motor centers are scattered throughout the rostral-caudal extent of the nucleus but collectively display a common set of electrophysiological properties. Additionally, we expect to find these LC projection neurons maintain an organized network of axon collaterals capable of supporting selective, synchronous release of NE in motor circuitries for the purpose of coordinately regulating operations across networks that are responsible for balance and movement dynamics. Investigation of this hypothesis will advance our knowledge of the role of the LC-NE system in motor control and provide a basis for treating movement disorders resulting from disease, injury, or normal aging.
Keywords: TrAC; locus coeruleus; motor centers; norepinephrine; viral vector track tracing.
Copyright © 2022 Waterhouse, Predale, Plummer, Jensen and Chandler.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer SB declared a past co-authorship with the authors BW and DC to the handling editor.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

