Alleviation of cisplatin-induced neuropathic pain, neuronal apoptosis, and systemic inflammation in mice by rapamycin
- PMID: 36248001
- PMCID: PMC9554141
- DOI: 10.3389/fnagi.2022.891593
Alleviation of cisplatin-induced neuropathic pain, neuronal apoptosis, and systemic inflammation in mice by rapamycin
Abstract
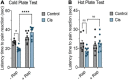
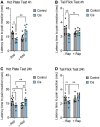
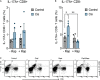
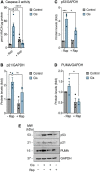
Platinum-based chemotherapeutic treatment of cancer patients is associated with debilitating adverse effects. Several adverse effects have been well investigated, and can be managed satisfactorily, but chemotherapy-induced peripheral neuropathy (CIPN) remains poorly treated. Our primary aim in this study was to investigate the neuroprotective effect of the immunomodulatory drug rapamycin in the mitigation of cisplatin-induced neurotoxicity. Pain assays were performed in vivo to determine whether rapamycin would prevent or significantly decrease cisplatin-induced neurotoxicity in adult male Balb/c mice. Neuropathic pain induced by both chronic and acute exposure to cisplatin was measured by hot plate assay, cold plate assay, tail-flick test, and plantar test. Rapamycin co-treatment resulted in significant reduction in cisplatin-induced nociceptive-like symptoms. To understand the underlying mechanisms behind rapamycin-mediated neuroprotection, we investigated its effect on certain inflammatory mediators implicated in the propagation of chemotherapy-induced neurotoxicity. Interestingly, cisplatin was found to significantly increase peripheral IL-17A expression and CD8- T cells, which were remarkably reversed by the pre-treatment of mice with rapamycin. In addition, rapamycin reduced the cisplatin-induced neuronal apoptosis marked by decreased neuronal caspase-3 activity. The rapamycin neuroprotective effect was also associated with reversal of the changes in protein expression of p21Cip1, p53, and PUMA. Collectively, rapamycin alleviated some features of cisplatin-induced neurotoxicity in mice and can be further investigated for the treatment of cisplatin-induced peripheral neuropathy.
Keywords: CIPN; IL-17A; cisplatin; p21; rapamycin.
Copyright © 2022 Alotaibi, Al-Aqil, Alqahtani, Alanazi, Nadeem, Ahmad, Lapresa, Alharbi, Alshammari, Alotaibi, Saleh and Alrowis.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Thymoquinone prevents cisplatin neurotoxicity in primary DRG neurons.Neurotoxicology. 2018 Dec;69:68-76. doi: 10.1016/j.neuro.2018.09.001. Epub 2018 Sep 15. Neurotoxicology. 2018. PMID: 30227172
-
Inhibition of Mitochondrial p53 Accumulation by PFT-μ Prevents Cisplatin-Induced Peripheral Neuropathy.Front Mol Neurosci. 2017 Apr 18;10:108. doi: 10.3389/fnmol.2017.00108. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28458631 Free PMC article.
-
Depletion of senescent-like neuronal cells alleviates cisplatin-induced peripheral neuropathy in mice.Sci Rep. 2020 Aug 25;10(1):14170. doi: 10.1038/s41598-020-71042-6. Sci Rep. 2020. PMID: 32843706 Free PMC article.
-
Neuroinflammatory Process Involved in Different Preclinical Models of Chemotherapy-Induced Peripheral Neuropathy.Front Immunol. 2021 Feb 4;11:626687. doi: 10.3389/fimmu.2020.626687. eCollection 2020. Front Immunol. 2021. PMID: 33613570 Free PMC article. Review.
-
Chemotherapy-induced peripheral neuropathy: part 1-current state of knowledge and perspectives for pharmacotherapy.Pharmacol Rep. 2020 Jun;72(3):486-507. doi: 10.1007/s43440-020-00109-y. Epub 2020 May 11. Pharmacol Rep. 2020. PMID: 32394362 Free PMC article. Review.
Cited by
-
Neuroprotective Effects of Trimetazidine against Cisplatin-Induced Peripheral Neuropathy: Involvement of AMPK-Mediated PI3K/mTOR, Nrf2, and NF-κB Signaling Axes.Oxid Med Cell Longev. 2024 Aug 20;2024:6612009. doi: 10.1155/2024/6612009. eCollection 2024. Oxid Med Cell Longev. 2024. PMID: 39502494 Free PMC article.
-
Kinin B1 and B2 Receptors Contribute to Cisplatin-Induced Painful Peripheral Neuropathy in Male Mice.Pharmaceutics. 2023 Mar 6;15(3):852. doi: 10.3390/pharmaceutics15030852. Pharmaceutics. 2023. PMID: 36986713 Free PMC article.
-
Nucleoside Reverse Transcriptase Inhibitor (NRTI)-Induced Neuropathy and Mitochondrial Toxicity: Limitations of the Poly-γ Hypothesis and the Potential Roles of Autophagy and Drug Transport.Pharmaceutics. 2024 Dec 13;16(12):1592. doi: 10.3390/pharmaceutics16121592. Pharmaceutics. 2024. PMID: 39771570 Free PMC article. Review.
-
Effects of Repeated Cisplatin and Monosodium Glutamate on Visceral Sensitivity in Rats.Cells. 2024 Dec 30;14(1):26. doi: 10.3390/cells14010026. Cells. 2024. PMID: 39791727 Free PMC article.
-
Monoclonal Antibody Targeting CGRP Relieves Cisplatin-Induced Neuropathic Pain by Attenuating Neuroinflammation.Neurotox Res. 2024 Jan 9;42(1):8. doi: 10.1007/s12640-023-00685-w. Neurotox Res. 2024. PMID: 38194189
References
-
- Ahmad S. F., Ansari M. A., Nadeem A., Bakheet S. A., Alshammari M. A., Attia S. M. (2018). Protection by tyrosine kinase inhibitor, tyrphostin AG126, through the suppression of IL-17A, RORγt, and T-bet signaling, in the BTBR mouse model of autism. Brain Res. Bull. 142 328–337. 10.1016/j.brainresbull.2018.08.020 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

